Inhibitory efficiency of Andrographis paniculata extract on viral multiplication and nitric oxide production
et al., Scientific Reports, doi:10.1038/s41598-023-46249-y, Nov 2023
In vitro study analyzing the antiviral and anti-inflammatory properties of extracts and fractions from Andrographis paniculata, against herpes simplex virus-1 (HSV-1) and influenza A virus H3N2 as surrogates for COVID-19. The results showed that certain A. paniculata extracts, particularly the andrographolide-rich fractions, could directly inactivate both viruses and inhibit their replication in infected cells. Interestingly, one andrographolide-free fraction also displayed potent antiviral effects against both viruses. Additionally, specific extracts inhibited nitric oxide production, highlighting their anti-inflammatory potential. The antiviral and anti-inflammatory activities demonstrated in this study suggest that further exploration of A. paniculata extracts as potential treatments for COVID-19 may be warranted.
25 preclinical studies support the efficacy of andrographolide for COVID-19:
In vitro studies demonstrate inhibition of the MproA,18 protein.
In vitro studies demonstrate efficacy in Calu-3B,18, A549C,14, and HUVECD,18 cells.
Animal studies demonstrate efficacy in Sprague Dawley miceE,18 and Golden Syrian hamstersF,14.
Andrographolide inhibits Mpro in a dose-dependent manner18, reduces ACE2 levels in the lung tissue of mice in combination with baicalein18, inhibits binding between the SARS-CoV-2 spike protein and ACE218, alleviates lung inflammation and cytokine storm in mice18, and improves survival and reduces lung inflammation via anti-inflammatory effects in Syrian hamsters14.
1.
Zhang et al., Effects and Mechanisms of Andrographolide for COVID-19: A Network Pharmacology-Based and Experimentally Validated Study, Natural Product Communications, doi:10.1177/1934578X241288428.
2.
Thomas et al., Cheminformatics approach to identify andrographolide derivatives as dual inhibitors of methyltransferases (nsp14 and nsp16) of SARS-CoV-2, Scientific Reports, doi:10.1038/s41598-024-58532-7.
3.
Arifin et al., Computational exploration of Andrographis paniculata herb compounds as potential antiviral agents targeting NSP3 (6W02) and NSP5 (7AR6) of SARS-COV-2, GSC Biological and Pharmaceutical Sciences, doi:10.30574/gscbps.2023.25.2.0292.
4.
Bhattarai et al., Investigating the binding affinity of andrographolide against human SARS-CoV-2 spike receptor-binding domain through docking and molecular dynamics simulations, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2023.2174596.
5.
Nguyen et al., The Potential of Ameliorating COVID-19 and Sequelae From Andrographis paniculata via Bioinformatics, Bioinformatics and Biology Insights, doi:10.1177/11779322221149622.
6.
Dassanayake et al., Molecular Docking and In-Silico Analysis of Natural Biomolecules against Dengue, Ebola, Zika, SARS-CoV-2 Variants of Concern and Monkeypox Virus, International Journal of Molecular Sciences, doi:10.3390/ijms231911131.
7.
Ningrum et al., Potency Of Andrographolide, L-Mimosine And Asiaticoside Compound As Antiviral For Covid-19 Based On In Silico Method, Proceedings Universitas Muhammadiyah Yogyakarta Undergraduate Conference, doi:10.18196/umygrace.v2i2.418.
8.
Ravichandran et al., Identification of Potential Semisynthetic Andrographolide Derivatives to Combat COVID-19 by Targeting the SARS-COV-2 Spike Protein and Human ACE2 Receptor– An In-silico Approach, Biointerface Research in Applied Chemistry, doi:10.33263/BRIAC132.155.
9.
Saeheng et al., In Silico Prediction of Andrographolide Dosage Regimens for COVID-19 Treatment, The American Journal of Chinese Medicine, doi:10.1142/S0192415X22500732.
10.
Khanal et al., Combination of system biology to probe the anti-viral activity of andrographolide and its derivative against COVID-19, RSC Advances, doi:10.1039/D0RA10529E.
11.
Rehan et al., A Computational Approach Identified Andrographolide as a Potential Drug for Suppressing COVID-19-Induced Cytokine Storm, Frontiers in Immunology, doi:10.3389/fimmu.2021.648250.
12.
Rajagopal et al., Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-020-00126-x.
13.
Dey et al., The role of andrographolide and its derivative in COVID-19 associated proteins and immune system, Research Square, doi:10.21203/rs.3.rs-35800/v1.
14.
Kongsomros et al., In vivo evaluation of Andrographis paniculata and Boesenbergia rotunda extract activity against SARS-CoV-2 Delta variant in Golden Syrian hamsters: Potential herbal alternative for COVID-19 treatment, Journal of Traditional and Complementary Medicine, doi:10.1016/j.jtcme.2024.05.004.
15.
Chaopreecha et al., Andrographolide attenuates SARS-CoV-2 infection via an up-regulation of glutamate-cysteine ligase catalytic subunit (GCLC), Phytomedicine, doi:10.1016/j.phymed.2024.156279.
16.
Li et al., Andrographolide suppresses SARS-CoV-2 infection by downregulating ACE2 expression: A mechanistic study, Antiviral Therapy, doi:10.1177/13596535241259952.
17.
Low et al., The wide spectrum anti-inflammatory activity of andrographolide in comparison to NSAIDs: a promising therapeutic compound against the cytokine storm, bioRxiv, doi:10.1101/2024.02.21.581396.
18.
Wan et al., Synergistic inhibition effects of andrographolide and baicalin on coronavirus mechanisms by downregulation of ACE2 protein level, Scientific Reports, doi:10.1038/s41598-024-54722-5.
19.
Siridechakorn et al., Inhibitory efficiency of Andrographis paniculata extract on viral multiplication and nitric oxide production, Scientific Reports, doi:10.1038/s41598-023-46249-y.
a.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
b.
Calu-3 is a human lung adenocarcinoma cell line with moderate ACE2 and TMPRSS2 expression and SARS-CoV-2 susceptibility. It provides a model of the human respiratory epithelium, but many not be ideal for modeling early stages of infection due to the moderate expression levels of ACE2 and TMPRSS2.
c.
A549 is a human lung carcinoma cell line with low ACE2 expression and SARS-CoV-2 susceptibility. Viral entry/replication can be studied but the cells may not replicate all aspects of lung infection.
d.
HUVEC (Human Umbilical Vein Endothelial Cells) are primary endothelial cells derived from the vein of the umbilical cord. They are used to study vascular biology, including inflammation, angiogenesis, and viral interactions with endothelial cells.
e.
An outbred multipurpose breed of albino mouse used extensively in medical research.
f.
A rodent model widely used in infectious disease research due to their susceptibility to viral infections and similar disease progression to humans.
Siridechakorn et al., 13 Nov 2023, Thailand, peer-reviewed, 9 authors.
Contact: bhparvapan@gmail.com, netiw@nu.ac.th.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Inhibitory efficiency of Andrographis paniculata extract on viral multiplication and nitric oxide production
Scientific Reports, doi:10.1038/s41598-023-46249-y
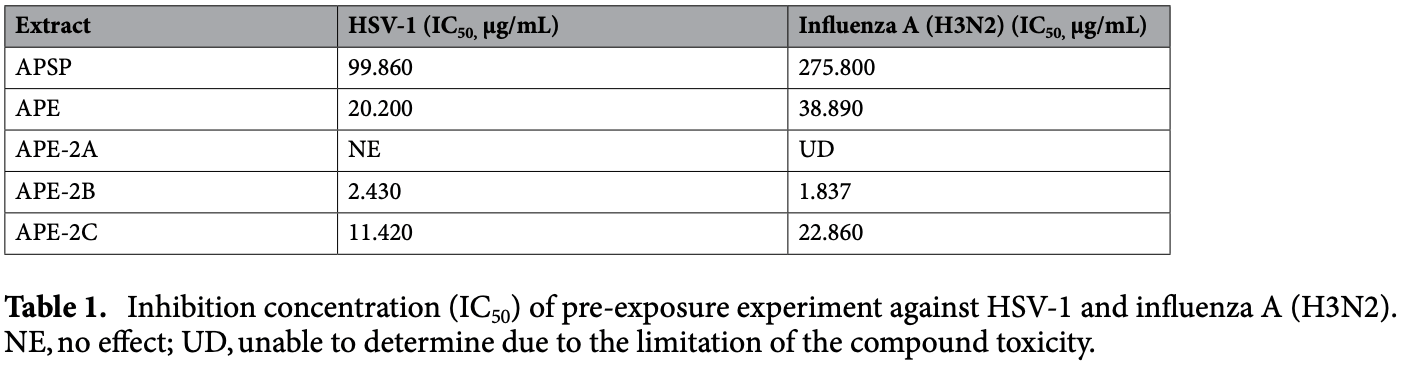
Andrographis paniculata (Burm. F.) Nees is a medicinal plant previously reported with broad-spectrum antivirals but the mode of inhibition remains elusive. The objective of this study was to identify the most active fraction from A. paniculata ethanol extract (APE, APE-2A, APE-2B and APE-2C) and dry powder extract (APSP) against influenza A (H3N2), representing RNA viruses, and herpes simplex virus-1 (HSV-1), representing DNA viruses. The results showed that the fractions APSP, APE, APE-2B, and APE-2C directly neutralized the HSV-1 and influenza A (H3N2) when incubated at room temperature for 60 min before infecting the cells. The results also showed that the additional APE-2A fraction also directly neutralized the influenza A (H3N2), but not the HSV-1. The APE, APE-2B and APE-2C inhibited the HSV-1 by more than 0.5 log when the fractions were introduced after infection. Similarly, the APSP and APE inhibited the influenza A (H3N2) more than 0.5 log after infection. Only 50 μg/mL APE-2C inhibited the viruses greater than 0.5 log. In addition, A. paniculata extracts were also evaluated for their interfering capacities against nitric oxide (NO) production in LPS-activated RAW 264.7 macrophages. As well, APE-2C potently inhibited NO production at the IC 50 of 6.08 μg/ mL. HPLC and LC-MS analysis indicated that the most actively antiviral fractions did not contain any andrographolide derivatives, whereas the andrographolide-rich fractions showed moderate activity. Andrographis paniculata (Burm. F.) Nees, commonly known in Thailand as "Fah Thalai Jone" belongs to the Acanthaceae family and is found throughout tropical and subtropical Asia and Southeast Asia 1 . A. paniculata extracts exhibit a wide range of pharmacological activities such as immunostimulatory 1,2 , antiviral 3,4 , and antibacterial activities 5 . A. paniculata extracts contain several constituents with a high content of andrographolide. The extract has broad-spectrum antiviral properties including the possibility of in vitro and in vivo anti-HIV 6 , as well as in vitro anti-dengue virus and chikungunya virus activity 7 . A. paniculata extracts are effective against the herpes simplex virus type 1 (HSV-1) that causes herpes 8 as well as reduced the inflammation caused by influenza viruses 9,10 . In addition, these extracts have also been reported to inhibit the division of influenza viruses 11 , hepatitis C virus 12 , and anti-viral mutations that cause resistance to such antiretroviral drugs 13 . The anti-inflammatory 14 and anti-allergic activities 15 of A. paniculata have been attributed to andrographolide, which is the major active compound 16,17 . Because of the COVID-19 pandemic that first arose in December 2019, this virus poses a serious risk to patients. The key mechanism for the manifestation of this disease is the inflammation process, which was the focus of this research which investigated the antiviral efficacy and anti-inflammation of A. paniculata extract. As..
Author contributions
Competing interests The authors declare no competing interests.
References
Abu-Ghefreh, Canatan, Ezeamuzie, In vitro and in vivo anti-inflammatory effects of andrographolide, Int. Immunopharmacol, doi:10.1016/j.intimp.2008.12.002
Aromdee, Suebsasana, Ekalaksananan, Pientong, Thongchai, Stage of action of naturally occurring andrographolides and their semisynthetic analogues against herpes simplex virus type 1 in vitro, Planta Med, doi:10.1055/s-0030-1250659
Cai, 14-Deoxy-11,12-dehydroandrographolide exerts anti-influenza A virus activity and inhibits replication of H5N1 virus by restraining nuclear export of viral ribonucleoprotein complexes, Antivir. Res, doi:10.1016/j.antiviral.2015.03.008
Cai, 14-Deoxy-11,12-didehydroandrographolide attenuates excessive inflammatory responses and protects mice lethally challenged with highly pathogenic A(H5N1) influenza viruses, Antivir. Res, doi:10.1016/j.antiviral.2016.07.020
Calabrese, A phase I trial of andrographolide in HIV positive patients and normal volunteers, Phytother. Res, doi:10.1002/1099-1573(200008)14:5%3c333::aid-ptr584%3e3.0.co;2-d
Chanchal-Garg, Saurabh, Munish, Stability indicating studies of Andrographis paniculata extract by validate HPTLC protocol, J. Pharmacogn. Phytochem
Chandramohan, Kaphle, Chekuri, Gangarudraiah, Siddaiah, Evaluating andrographolide as a potent inhibitor of NS3-4A protease and its drug-resistant mutants using in silico approaches, Adv. Virol, doi:10.1155/2015/972067
Chen, Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro, Biol. Pharm. Bull, doi:10.1248/bpb.32.1385
Ding, Andrographolide inhibits influenza A virus-induced inflammation in a murine model through NF-kappa B and JAK-STAT signaling pathway, Microbes Infect, doi:10.1016/j.micinf.2017.08.009
Feng, Wang, Ma, Li, Zhao, A potential in vitro and in vivo anti-HIV drug screening system for Chinese herbal medicines, Phytother. Res, doi:10.1002/ptr.3658
Jadhav, Karuppayil, Andrographis Paniculata (burm, Wall ex Nees: Antiviral properties, Phytother. Res, doi:10.1002/ptr.7145
Jain, Antiviral activity of ethanolic extract of Nilavembu kudineer against dengue and chikungunya virus through in vitro evaluation, J. Ayurveda Integr. Med, doi:10.1016/j.jaim.2018.05.006
Jang, Anti-inflammatory activity of eudesmane-type sesquiterpenoids from salvia plebeia, J. Nat. Prod, doi:10.1021/acs.jnatprod.7b00326
Kumar, Sridevi, Kumar, Nanduri, Rajagopal, Anticancer and immunostimulatory compounds from Andrographis paniculata, J. Ethnopharmacol, doi:10.1016/j.jep.2004.03.004
Lee, Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/ Nrf2 pathway in human hepatoma cells, Br. J. Pharmacol, doi:10.1111/bph.12440
Madav, Tandan, Dinesh-Kumar, Anti-allergic activity of andrographolide, Indian J. Pharm. Sci
Madav, Tandan, Lal, Tripathi, Anti-inflammatory activity of andrographolide, Fitoterapia
Rajagopal, Kumar, Deevi, Satyanarayana, Rajagopalan et al., a potential cancer therapeutic agent isolated from Andrographis paniculata, J. Exp. Ther. Oncol, doi:10.1046/j.1359-4117.2003.01090.x
Sarigaputi, Sommit, Teerawatananond, Pudhom, Weakly Anti-inflammatory Limonoids from the Seeds of Xylocarpus rumphii, Scientific Reports, doi:10.1021/np5003687
Seubsasana, Pientong, Ekalaksananan, Thongchai, Aromdee, A potential andrographolide analogue against the replication of herpes simplex virus type 1 in vero cells, Med. Chem, doi:10.2174/157340611795564268
Sheeja, Kuttan, Effect of andrographis paniculata as an adjuvant in combined chemo-radio and whole body hyperthermia treatment-a preliminary study, Immunopharmacol. Immunotoxicol, doi:10.1080/08923970701692916
Singha, Roy, Dey, Antimicrobial activity of Andrographis paniculata, Fitoterapia, doi:10.1016/s0367-326X(03)00159-X
DOI record:
{
"DOI": "10.1038/s41598-023-46249-y",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-023-46249-y",
"abstract": "<jats:title>Abstract</jats:title><jats:p><jats:italic>Andrographis paniculata</jats:italic> (Burm. F.) Nees is a medicinal plant previously reported with broad-spectrum antivirals but the mode of inhibition remains elusive. The objective of this study was to identify the most active fraction from <jats:italic>A. paniculata</jats:italic> ethanol extract (APE, APE-2A, APE-2B and APE-2C) and dry powder extract (APSP) against influenza A (H3N2), representing RNA viruses, and herpes simplex virus-1 (HSV-1), representing DNA viruses. The results showed that the fractions APSP, APE, APE-2B, and APE-2C directly neutralized the HSV-1 and influenza A (H3N2) when incubated at room temperature for 60 min before infecting the cells. The results also showed that the additional APE-2A fraction also directly neutralized the influenza A (H3N2), but not the HSV-1. The APE, APE-2B and APE-2C inhibited the HSV-1 by more than 0.5 log when the fractions were introduced after infection. Similarly, the APSP and APE inhibited the influenza A (H3N2) more than 0.5 log after infection. Only 50 μg/mL APE-2C inhibited the viruses greater than 0.5 log. In addition, A. <jats:italic>paniculata</jats:italic> extracts were also evaluated for their interfering capacities against nitric oxide (NO) production in LPS-activated RAW 264.7 macrophages. As well, APE-2C potently inhibited NO production at the IC<jats:sub>50</jats:sub> of 6.08 μg/mL. HPLC and LC–MS analysis indicated that the most actively antiviral fractions did not contain any andrographolide derivatives, whereas the andrographolide-rich fractions showed moderate activity.</jats:p>",
"alternative-id": [
"46249"
],
"article-number": "19738",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "25 October 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "30 October 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "13 November 2023"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Siridechakorn",
"given": "Ittipon",
"sequence": "first"
},
{
"affiliation": [],
"family": "Bhattarakosol",
"given": "Parvapan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sasivimolrattana",
"given": "Thanayod",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anoma",
"given": "Sasiprapa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wongwad",
"given": "Eakkaluk",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nuengchamnong",
"given": "Nitra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kowitdamrong",
"given": "Ekasit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boonyasuppayakorn",
"given": "Siwaporn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Waranuch",
"given": "Neti",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2023,
11,
13
]
],
"date-time": "2023-11-13T10:01:54Z",
"timestamp": 1699869714000
},
"deposited": {
"date-parts": [
[
2023,
11,
13
]
],
"date-time": "2023-11-13T11:05:20Z",
"timestamp": 1699873520000
},
"funder": [
{
"DOI": "10.13039/501100005076",
"award": [
"CRP6405032190"
],
"doi-asserted-by": "publisher",
"name": "Agricultural Research Development Agency"
},
{
"DOI": "10.13039/100015396",
"award": [
"TC-A 16/63"
],
"doi-asserted-by": "publisher",
"name": "Thailand Center of Excellence for Life Sciences"
},
{
"award": [
"RA-MF-20/64"
],
"name": "Ratchadapiseksompotch endowment funds, Faculty of Medicine"
},
{
"DOI": "10.13039/501100014795",
"doi-asserted-by": "publisher",
"name": "Center of Excellence for Innovation in Chemistry"
}
],
"indexed": {
"date-parts": [
[
2023,
11,
14
]
],
"date-time": "2023-11-14T00:33:37Z",
"timestamp": 1699922017998
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
11,
13
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
13
]
],
"date-time": "2023-11-13T00:00:00Z",
"timestamp": 1699833600000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
13
]
],
"date-time": "2023-11-13T00:00:00Z",
"timestamp": 1699833600000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-023-46249-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-023-46249-y",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-023-46249-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2023,
11,
13
]
]
},
"published-online": {
"date-parts": [
[
2023,
11,
13
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.jep.2004.03.004",
"author": "RA Kumar",
"doi-asserted-by": "publisher",
"first-page": "291",
"journal-title": "J. Ethnopharmacol.",
"key": "46249_CR1",
"unstructured": "Kumar, R. A., Sridevi, K., Kumar, N. V., Nanduri, S. & Rajagopal, S. Anticancer and immunostimulatory compounds from Andrographis paniculata. J. Ethnopharmacol. 92, 291–295. https://doi.org/10.1016/j.jep.2004.03.004 (2004).",
"volume": "92",
"year": "2004"
},
{
"DOI": "10.1046/j.1359-4117.2003.01090.x",
"author": "S Rajagopal",
"doi-asserted-by": "publisher",
"first-page": "147",
"journal-title": "J. Exp. Ther. Oncol.",
"key": "46249_CR2",
"unstructured": "Rajagopal, S., Kumar, R. A., Deevi, D. S., Satyanarayana, C. & Rajagopalan, R. Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J. Exp. Ther. Oncol. 3, 147–158. https://doi.org/10.1046/j.1359-4117.2003.01090.x (2003).",
"volume": "3",
"year": "2003"
},
{
"DOI": "10.1002/1099-1573(200008)14:5<333::aid-ptr584>3.0.co;2-d",
"author": "C Calabrese",
"doi-asserted-by": "publisher",
"first-page": "333",
"journal-title": "Phytother. Res.",
"key": "46249_CR3",
"unstructured": "Calabrese, C. et al. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother. Res. 14, 333–338. https://doi.org/10.1002/1099-1573(200008)14:5%3c333::aid-ptr584%3e3.0.co;2-d (2000).",
"volume": "14",
"year": "2000"
},
{
"DOI": "10.1002/ptr.7145",
"author": "AK Jadhav",
"doi-asserted-by": "publisher",
"first-page": "5365",
"journal-title": "Phytother. Res.",
"key": "46249_CR4",
"unstructured": "Jadhav, A. K. & Karuppayil, S. M. Andrographis paniculata (Burm. F) Wall ex Nees: Antiviral properties. Phytother. Res. 35, 5365–5373. https://doi.org/10.1002/ptr.7145 (2021).",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/s0367-326X(03)00159-X",
"author": "PK Singha",
"doi-asserted-by": "publisher",
"first-page": "692",
"journal-title": "Fitoterapia",
"key": "46249_CR5",
"unstructured": "Singha, P. K., Roy, S. & Dey, S. Antimicrobial activity of Andrographis paniculata. Fitoterapia 74, 692–694. https://doi.org/10.1016/s0367-326X(03)00159-X (2003).",
"volume": "74",
"year": "2003"
},
{
"DOI": "10.1002/ptr.3658",
"author": "L Feng",
"doi-asserted-by": "publisher",
"first-page": "899",
"journal-title": "Phytother. Res.",
"key": "46249_CR6",
"unstructured": "Feng, L., Wang, L., Ma, Y. Y., Li, M. & Zhao, G. Q. A potential in vitro and in vivo anti-HIV drug screening system for Chinese herbal medicines. Phytother. Res. 26, 899–907. https://doi.org/10.1002/ptr.3658 (2012).",
"volume": "26",
"year": "2012"
},
{
"DOI": "10.1016/j.jaim.2018.05.006",
"author": "J Jain",
"doi-asserted-by": "publisher",
"first-page": "329",
"journal-title": "J. Ayurveda Integr. Med.",
"key": "46249_CR7",
"unstructured": "Jain, J. et al. Antiviral activity of ethanolic extract of Nilavembu kudineer against dengue and chikungunya virus through in vitro evaluation. J. Ayurveda Integr. Med. 11, 329–335. https://doi.org/10.1016/j.jaim.2018.05.006 (2020).",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1055/s-0030-1250659",
"author": "C Aromdee",
"doi-asserted-by": "publisher",
"first-page": "915",
"journal-title": "Planta Med.",
"key": "46249_CR8",
"unstructured": "Aromdee, C., Suebsasana, S., Ekalaksananan, T., Pientong, C. & Thongchai, S. Stage of action of naturally occurring andrographolides and their semisynthetic analogues against herpes simplex virus type 1 in vitro. Planta Med. 77, 915–921. https://doi.org/10.1055/s-0030-1250659 (2011).",
"volume": "77",
"year": "2011"
},
{
"DOI": "10.1016/j.antiviral.2016.07.020",
"author": "WT Cai",
"doi-asserted-by": "publisher",
"first-page": "95",
"journal-title": "Antivir. Res.",
"key": "46249_CR9",
"unstructured": "Cai, W. T. et al. 14-Deoxy-11,12-didehydroandrographolide attenuates excessive inflammatory responses and protects mice lethally challenged with highly pathogenic A(H5N1) influenza viruses. Antivir. Res. 133, 95–105. https://doi.org/10.1016/j.antiviral.2016.07.020 (2016).",
"volume": "133",
"year": "2016"
},
{
"DOI": "10.1016/j.micinf.2017.08.009",
"author": "Y Ding",
"doi-asserted-by": "publisher",
"first-page": "605",
"journal-title": "Microbes Infect.",
"key": "46249_CR10",
"unstructured": "Ding, Y. et al. Andrographolide inhibits influenza A virus-induced inflammation in a murine model through NF-kappa B and JAK-STAT signaling pathway. Microbes Infect. 19, 605–615. https://doi.org/10.1016/j.micinf.2017.08.009 (2017).",
"volume": "19",
"year": "2017"
},
{
"DOI": "10.1016/j.antiviral.2015.03.008",
"author": "WT Cai",
"doi-asserted-by": "publisher",
"first-page": "82",
"journal-title": "Antivir. Res.",
"key": "46249_CR11",
"unstructured": "Cai, W. T. et al. 14-Deoxy-11,12-dehydroandrographolide exerts anti-influenza A virus activity and inhibits replication of H5N1 virus by restraining nuclear export of viral ribonucleoprotein complexes. Antivir. Res. 118, 82–92. https://doi.org/10.1016/j.antiviral.2015.03.008 (2015).",
"volume": "118",
"year": "2015"
},
{
"DOI": "10.1111/bph.12440",
"author": "JC Lee",
"doi-asserted-by": "publisher",
"first-page": "237",
"journal-title": "Br. J. Pharmacol.",
"key": "46249_CR12",
"unstructured": "Lee, J. C. et al. Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cells. Br. J. Pharmacol. 171, 237–252. https://doi.org/10.1111/bph.12440 (2014).",
"volume": "171",
"year": "2014"
},
{
"DOI": "10.1155/2015/972067",
"author": "V Chandramohan",
"doi-asserted-by": "publisher",
"journal-title": "Adv. Virol.",
"key": "46249_CR13",
"unstructured": "Chandramohan, V., Kaphle, A., Chekuri, M., Gangarudraiah, S. & Siddaiah, G. B. Evaluating andrographolide as a potent inhibitor of NS3–4A protease and its drug-resistant mutants using in silico approaches. Adv. Virol. https://doi.org/10.1155/2015/972067 (2015).",
"year": "2015"
},
{
"DOI": "10.1080/08923970701692916",
"author": "K Sheeja",
"doi-asserted-by": "publisher",
"first-page": "181",
"journal-title": "Immunopharmacol. Immunotoxicol.",
"key": "46249_CR14",
"unstructured": "Sheeja, K. & Kuttan, G. Effect of andrographis paniculata as an adjuvant in combined chemo-radio and whole body hyperthermia treatment—a preliminary study. Immunopharmacol. Immunotoxicol. 30, 181–194. https://doi.org/10.1080/08923970701692916 (2008).",
"volume": "30",
"year": "2008"
},
{
"author": "HCT Madav",
"first-page": "176",
"journal-title": "Indian J. Pharm. Sci",
"key": "46249_CR15",
"unstructured": "Madav, H. C. T., Tandan, S. K. & Dinesh-Kumar, J. L. A. Anti-allergic activity of andrographolide. Indian J. Pharm. Sci 60, 176–718 (1998).",
"volume": "60",
"year": "1998"
},
{
"author": "S Madav",
"first-page": "452",
"journal-title": "Fitoterapia",
"key": "46249_CR16",
"unstructured": "Madav, S., Tandan, S. K., Lal, J. M. & Tripathi, H. C. Anti-inflammatory activity of andrographolide. Fitoterapia 67, 452–458 (1996).",
"volume": "67",
"year": "1996"
},
{
"DOI": "10.1016/j.intimp.2008.12.002",
"author": "AAA Abu-Ghefreh",
"doi-asserted-by": "publisher",
"first-page": "313",
"journal-title": "Int. Immunopharmacol.",
"key": "46249_CR17",
"unstructured": "Abu-Ghefreh, A. A. A., Canatan, H. & Ezeamuzie, C. I. In vitro and in vivo anti-inflammatory effects of andrographolide. Int. Immunopharmacol. 9, 313–318. https://doi.org/10.1016/j.intimp.2008.12.002 (2009).",
"volume": "9",
"year": "2009"
},
{
"DOI": "10.1021/acs.jnatprod.7b00326",
"author": "H-J Jang",
"doi-asserted-by": "publisher",
"first-page": "2666",
"journal-title": "J. Nat. Prod.",
"key": "46249_CR18",
"unstructured": "Jang, H.-J. et al. Anti-inflammatory activity of eudesmane-type sesquiterpenoids from salvia plebeia. J. Nat. Prod. 80, 2666–2676. https://doi.org/10.1021/acs.jnatprod.7b00326 (2017).",
"volume": "80",
"year": "2017"
},
{
"author": "PS Chanchal-Garg",
"first-page": "337",
"journal-title": "J. Pharmacogn. Phytochem.",
"key": "46249_CR19",
"unstructured": "Chanchal-Garg, P. S., Saurabh, S. & Munish, G. Stability indicating studies of Andrographis paniculata extract by validate HPTLC protocol. J. Pharmacogn. Phytochem. 5, 337–344 (2016).",
"volume": "5",
"year": "2016"
},
{
"DOI": "10.1248/bpb.32.1385",
"author": "J-X Chen",
"doi-asserted-by": "publisher",
"first-page": "1385",
"journal-title": "Biol. Pharm. Bull.",
"key": "46249_CR20",
"unstructured": "Chen, J.-X. et al. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biol. Pharm. Bull. 32, 1385–1391. https://doi.org/10.1248/bpb.32.1385 (2009).",
"volume": "32",
"year": "2009"
},
{
"DOI": "10.2174/157340611795564268",
"author": "S Seubsasana",
"doi-asserted-by": "publisher",
"first-page": "237",
"journal-title": "Med. Chem.",
"key": "46249_CR21",
"unstructured": "Seubsasana, S., Pientong, C., Ekalaksananan, T., Thongchai, S. & Aromdee, C. A potential andrographolide analogue against the replication of herpes simplex virus type 1 in vero cells. Med. Chem. 7, 237–244. https://doi.org/10.2174/157340611795564268 (2011).",
"volume": "7",
"year": "2011"
},
{
"DOI": "10.1021/np5003687",
"author": "C Sarigaputi",
"doi-asserted-by": "publisher",
"first-page": "2037",
"journal-title": "J. Nat. Prod.",
"key": "46249_CR22",
"unstructured": "Sarigaputi, C., Sommit, D., Teerawatananond, T. & Pudhom, K. Weakly Anti-inflammatory Limonoids from the Seeds of Xylocarpus rumphii. J. Nat. Prod. 77, 2037–2043. https://doi.org/10.1021/np5003687 (2014).",
"volume": "77",
"year": "2014"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-023-46249-y"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Inhibitory efficiency of Andrographis paniculata extract on viral multiplication and nitric oxide production",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "13"
}