Sigma Receptor Ligands Prevent COVID Mortality In Vivo: Implications for Future Therapeutics
et al., International Journal of Molecular Sciences, doi:10.3390/ijms242115718, Oct 2023
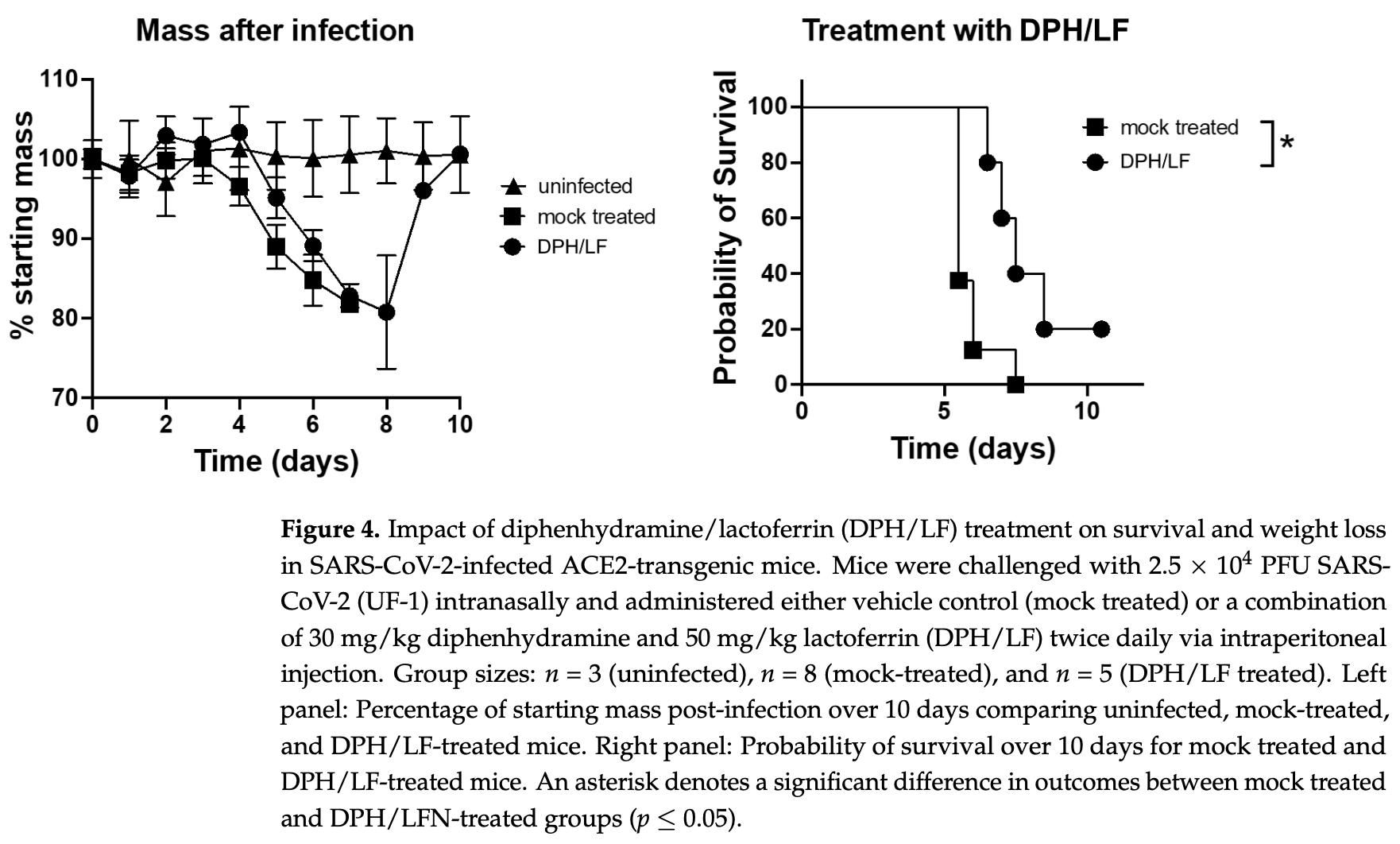
Mouse study showing the combination of lactoferrin and diphenhydramine resulted in slower weight loss, improved survival, and faster weight recovery in SARS-CoV-2 infected animals compared to controls. The study authors propose the lactoferrin/diphenhydramine combination as a promising, safe, cost-effective therapeutic candidate against SARS-CoV-2 and future coronaviruses, warranting further clinical investigation to determine efficacy for COVID-19 patients. The components target different viral life cycle mechanisms and have established safety records. In addition to lactoferrin and diphenhydramine, the study tested two highly specific sigma receptor ligands - CM398 (sigma-2 specific) and AZ66 (sigma-1 and sigma-2), with CM398 showing improved results.
18 preclinical studies support the efficacy of lactoferrin for COVID-19:
Study covers lactoferrin and antihistamine H1RAs.
1.
da Silva et al., Immunomodulatory effect of bovine lactoferrin during SARS-CoV-2 infection, Frontiers in Immunology, doi:10.3389/fimmu.2024.1456634.
2.
Cutone et al., Lactoferrin binding to Sars-CoV-2 Spike glycoprotein protects host from infection, inflammation and iron dysregulation., Research Square, doi:10.21203/rs.3.rs-1605740/v1.
3.
Miotto et al., Molecular Mechanisms Behind Anti SARS-CoV-2 Action of Lactoferrin, Frontiers in Molecular Biosciences, doi:10.3389/fmolb.2021.607443.
4.
Babulic et al., Lactoferrin Binds through Its N-Terminus to the Receptor-Binding Domain of the SARS-CoV-2 Spike Protein, Pharmaceuticals, doi:10.3390/ph17081021.
5.
Yathindranath et al., Lipid Nanoparticle-Based Inhibitors for SARS-CoV-2 Host Cell Infection, International Journal of Nanomedicine, doi:10.2147/IJN.S448005.
6.
Alves et al., Inhibition of SARS-CoV-2 Infection in Vero Cells by Bovine Lactoferrin under Different Iron-Saturation States, Pharmaceuticals, doi:10.3390/ph16101352.
7.
Kobayashi-Sakamoto et al., Bovine lactoferrin suppresses the cathepsin-dependent pathway of SARS-CoV-2 entry in vitro, International Dairy Journal, doi:10.1016/j.idairyj.2023.105805.
8.
Andreu et al., Liposomal Lactoferrin Exerts Antiviral Activity against HCoV-229E and SARS-CoV-2 Pseudoviruses In Vitro, Viruses, doi:10.3390/v15040972.
9.
Yazawa et al., Evaluation of SARS-CoV-2 isolation in cell culture from nasal/nasopharyngeal swabs or saliva specimens of patients with COVID-19, Research Square, doi:10.21203/rs.3.rs-2676422/v1.
10.
Piacentini et al., Lactoferrin Inhibition of the Complex Formation between ACE2 Receptor and SARS CoV-2 Recognition Binding Domain, International Journal of Molecular Sciences, doi:10.3390/ijms23105436.
11.
Ostrov et al., Highly Specific Sigma Receptor Ligands Exhibit Anti-Viral Properties in SARS-CoV-2 Infected Cells, Pathogens, doi:10.3390/pathogens10111514.
12.
Mirabelli et al., Morphological cell profiling of SARS-CoV-2 infection identifies drug repurposing candidates for COVID-19, Proceedings of the National Academy of Sciences, doi:10.1073/pnas.2105815118.
13.
Salaris et al., Protective Effects of Lactoferrin against SARS-CoV-2 Infection In Vitro, Nutrients, doi:10.3390/nu13020328.
Berkowitz et al., 29 Oct 2023, USA, peer-reviewed, 6 authors.
Contact: mhnorris@ufl.edu (corresponding author), reedberkowitz@ufl.edu, ostroda@pathology.ufl.edu.
Sigma Receptor Ligands Prevent COVID Mortality In Vivo: Implications for Future Therapeutics
International Journal of Molecular Sciences, doi:10.3390/ijms242115718
The emergence of lethal coronaviruses follows a periodic pattern which suggests a recurring cycle of outbreaks. It remains uncertain as to when the next lethal coronavirus will emerge, though its eventual emergence appears to be inevitable. New mutations in evolving SARS-CoV-2 variants have provided resistance to current antiviral drugs, monoclonal antibodies, and vaccines, reducing their therapeutic efficacy. This underscores the urgent need to investigate alternative therapeutic approaches. Sigma receptors have been unexpectedly linked to the SARS-CoV-2 life cycle due to the direct antiviral effect of their ligands. Coronavirus-induced cell stress facilitates the formation of an ER-derived complex conducive to its replication. Sigma receptor ligands are believed to prevent the formation of this complex. Repurposing FDA-approved drugs for COVID-19 offers a timely and cost-efficient strategy to find treatments with established safety profiles. Notably, diphenhydramine, a sigma receptor ligand, is thought to counteract the virus by inhibiting the creation of ER-derived replication vesicles. Furthermore, lactoferrin, a well-characterized immunomodulatory protein, has shown antiviral efficacy against SARS-CoV-2 both in laboratory settings and in living organisms. In the present study, we aimed to explore the impact of sigma receptor ligands on SARS-CoV-2-induced mortality in ACE2-transgenic mice. We assessed the effects of an investigational antiviral drug combination comprising a sigma receptor ligand and an immunomodulatory protein. Mice treated with sigma-2 receptor ligands or diphenhydramine and lactoferrin exhibited improved survival rates and rapid rebound in mass following the SARS-CoV-2 challenge compared to mock-treated animals. Clinical translation of these findings may support the discovery of new treatment and research strategies for SARS-CoV-2.
Institutional Review Board Statement: The studies and SOPs were approved by the University of Florida Institutional Biosafety Committee under protocol BIO5594. They were carried out in the University of Florida Environmental Health and Safety inspected and approved BSL3 and ABSL3 facilities. Ethical approval for the animal studies and procedures in this work were approved by the University of Florida IACUC under protocol #202111322. Humane endpoints and euthanasia guidelines according to the American Veterinary Medical Association were adhered to throughout the study.
Informed Consent
References
Banos, Lacasa, Spatio-Temporal Exploration of SARS Epidemic, doi:10.4000/cybergeo.12803
Campione, Lanna, Cosio, Rosa, Conte et al., Lactoferrin as Antiviral Treatment in COVID-19 Management: Preliminary Evidence, Int. J. Environ. Res. Public. Health, doi:10.3390/ijerph182010985
Cappanera, Palumbo, Kwan, Priante, Martella et al., When Does the Cytokine Storm Begin in COVID-19 Patients? A Quick Score to Recognize It, J. Clin. Med, doi:10.3390/jcm10020297
Charness, Gupta, Stack, Strymish, Adams et al., Rebound of SARS-CoV-2 Infection after Nirmatrelvir-Ritonavir Treatment, N. Engl. J. Med, doi:10.1056/NEJMc2206449
Cirino, Eans, Medina, Wilson, Mottinelli et al., Characterization of Sigma 1 Receptor Antagonist CM-304 and Its Analog, AZ-66: Novel Therapeutics Against Allodynia and Induced Pain, Front. Pharmacol, doi:10.3389/fphar.2019.00678
Crespo, Calleja, Fernández, Sacristan, Ruiz-Antorán et al., Real-World Effectiveness and Safety of Oral Combination Antiviral Therapy for Hepatitis C Virus Genotype 4 Infection, Clin. Gastroenterol. Hepatol, doi:10.1016/j.cgh.2017.02.020
Csr Mers Outbreaks, None
Fung, Huang, Liu, Coronavirus-Induced ER Stress Response and Its Involvement in Regulation of Coronavirus-Host Interactions, Virus Res, doi:10.1016/j.virusres.2014.09.016
Gordon, Jang, Bouhaddou, Xu, Obernier et al., A SARS-CoV-2 Protein Interaction Map Reveals Targets for Drug Repurposing, Nature, doi:10.1038/s41586-020-2286-9
Grodzki, Bluhm, Schaefer, Tagmount, Russo et al., Genome-Scale CRISPR Screens Identify Host Factors That Promote Human Coronavirus Infection, Genome Med, doi:10.1186/s13073-022-01013-1
Hayashi, The Sigma-1 Receptor in Cellular Stress Signaling, Front. Neurosci, doi:10.3389/fnins.2019.00733
Hu, Lewandowski, Tan, Zhang, Morgan et al., Naturally Occurring Mutations of SARS-CoV-2 Main Protease Confer Drug Resistance to Nirmatrelvir
Hu, Meng, Zhang, Xiang, Wang, The in Vitro Antiviral Activity of Lactoferrin against Common Human Coronaviruses and SARS-CoV-2 Is Mediated by Targeting the Heparan Sulfate Co-Receptor, Emerg. Microbes Infect, doi:10.1080/22221751.2021.1888660
Iketani, Liu, Guo, Liu, Chan et al., Antibody Evasion Properties of SARS-CoV-2 Omicron Sublineages, Nature, doi:10.1038/s41586-022-04594-4
Intagliata, Sharma, King, Mesangeau, Seminerio et al., Discovery of a Highly Selective Sigma-2 Receptor Ligand, 1-(4-(6,7-Dimethoxy-3,4-Dihydroisoquinolin-2(1H)-Yl)Butyl)-3-Methyl-1H-Benzo[d]Imidazol-2(3H)-One (CM398), with Drug-Like Properties and Antinociceptive Effects In Vivo, AAPS J, doi:10.1208/s12248-020-00472-x
James, Shen, Zavaleta, Nielsen, Mesangeau et al., New Positron Emission Tomography (PET) Radioligand for Imaging σ-1 Receptors in Living Subjects, J. Med. Chem, doi:10.1021/jm300371c
Jochmans, Liu, Donckers, Stoycheva, Boland et al., The Substitutions L50F, E166A and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance to Nirmatrelvir
Kabinger, Stiller, Schmitzová, Dienemann, Kokic et al., Mechanism of Molnupiravir-Induced SARS-CoV-2 Mutagenesis, Nat. Struct. Mol. Biol, doi:10.1038/s41594-021-00651-0
Kaufmann, Dorhoi, Hotchkiss, Bartenschlager, Host-Directed Therapies for Bacterial and Viral Infections, Nat. Rev. Drug Discov, doi:10.1038/nrd.2017.162
Kozlov, Merck's COVID Pill Loses Its Lustre: What That Means for the Pandemic, Nature, doi:10.1038/d41586-021-03667-0
Kozu, Iinuma, Ohashi, Saito, Akasu et al., Effect of Orally Administered Bovine Lactoferrin on the Growth of Adenomatous Colorectal Polyps in a Randomized, Placebo-Controlled Clinical Trial, Cancer Prev. Res, doi:10.1158/1940-6207.CAPR-08-0208
Lednicky, Waltzek, Mcgeehan, Loeb, Hamilton et al., Isolation and Genetic Characterization of Human Coronavirus NL63 in Primary Human Renal Proximal Tubular Epithelial Cells Obtained from a Commercial Supplier, and Confirmation of Its Replication in Two Different Types of Human Primary Kidney Cells, Virol. J, doi:10.1186/1743-422X-10-213
Liu, Wei, He, Differences in Case-Fatality-Rate of Emerging SARS-CoV-2 Variants, Public Health Pract, doi:10.1016/j.puhip.2022.100350
Matino, Tavella, Rizzi, Avanzi, Azzolina et al., Effect of Lactoferrin on Clinical Outcomes of Hospitalized Patients with COVID-19: The LAC Randomized Clinical Trial, Nutrients, doi:10.3390/nu15051285
Matthew, Leidner, Lockbaum, Henes, Zephyr et al., Drug Design Strategies to Avoid Resistance in Direct-Acting Antivirals and Beyond, Chem. Rev, doi:10.1021/acs.chemrev.0c00648
Merlos, Romero, Zamanillo, Plata-Salamán, Vela, Sigma-1 Receptor and Pain, Handb. Exp. Pharmacol, doi:10.1007/164_2017_9
Mihelc, Baker, Lanman, Coronavirus Infection Induces Progressive Restructuring of the Endoplasmic Reticulum Involving the Formation and Degradation of Double Membrane Vesicles, Virology, doi:10.1016/j.virol.2020.12.007
Mirabelli, Wotring, Zhang, Mccarty, Fursmidt et al., Morphological Cell Profiling of SARS-CoV-2 Infection Identifies Drug Repurposing Candidates for COVID-19, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2105815118
Moore, Anderson, Groom, Haridas, Baker, Three-Dimensional Structure of Diferric Bovine Lactoferrin at 2.8 Å Resolution, J. Mol. Biol, doi:10.1006/jmbi.1997.1386
Moreno, Perno, Mallon, Behrens, Corbeau et al., Two-Drug vs. Three-Drug Combinations for HIV-1: Do We Have Enough Data to Make the Switch?, HIV Med, doi:10.1111/hiv.12716
Naguib, Li, Ling, Grace, Nguyen-Viet et al., Live and Wet Markets: Food Access versus the Risk of Disease Emergence, Trends Microbiol, doi:10.1016/j.tim.2021.02.007
Navarro, Paredes, Tucto, Medina, Angles-Yanqui et al., Bovine Lactoferrin for the Prevention of COVID-19 Infection in Health Care Personnel: A Double-Blinded Randomized Clinical Trial (LF-COVID), BioMetals, doi:10.1007/s10534-022-00477-3
Okada, Tanaka, Sato, Ueno, Saito et al., Dose-Response Trial of Lactoferrin in Patients with Chronic Hepatitis C, Jpn. J. Cancer Res, doi:10.1111/j.1349-7006.2002.tb02484.x
Oladunni, Park, Pino, Gonzalez, Akhter et al., Lethality of SARS-CoV-2 Infection in K18 Human Angiotensin-Converting Enzyme 2 Transgenic Mice, Nat. Commun, doi:10.1038/s41467-020-19891-7
Ostrov, Bluhm, Li, Khan, Rohamare et al., Highly Specific Sigma Receptor Ligands Exhibit Anti-Viral Properties in SARS-CoV-2 Infected Cells, Pathogens, doi:10.3390/pathogens10111514
Petersen, Koopmans, Go, Hamer, Petrosillo et al., Comparing SARS-CoV-2 with SARS-CoV and Influenza Pandemics, Lancet Infect. Dis, doi:10.1016/S1473-3099(20)30484-9
Piacentini, Centi, Miotto, Milanetti, Di Rienzo et al., Lactoferrin Inhibition of the Complex Formation between ACE2 Receptor and SARS CoV-2 Recognition Binding Domain, Int. J. Mol. Sci, doi:10.3390/ijms23105436
Presti, Manti, Parisi, Papale, Barbagallo et al., Lactoferrin: Cytokine Modulation and Application in Clinical Practice, J. Clin. Med, doi:10.3390/jcm10235482
Rathnasinghe, Strohmeier, Amanat, Gillespie, Krammer et al., Comparison of Transgenic and Adenovirus hACE2 Mouse Models for SARS-CoV-2 Infection, Emerg. Microbes Infect, doi:10.1080/22221751.2020.1838955
Reznikov, Norris, Vashisht, Bluhm, Li et al., Identification of Antiviral Antihistamines for COVID-19 Repurposing, Biochem. Biophys. Res. Commun, doi:10.1016/j.bbrc.2020.11.095
Ricciardi, Guarino, Giaquinto, Polishchuk, Santoro et al., The Role of NSP6 in the Biogenesis of the SARS-CoV-2 Replication Organelle, Nature, doi:10.1038/s41586-022-04835-6
Rosa, Tripepi, Naldi, Aimati, Santangeli et al., Ambulatory COVID-19 Patients Treated with Lactoferrin as a Supplementary Antiviral Agent: A Preliminary Study, J. Clin. Med, doi:10.3390/jcm10184276
Sambo, Lebowitz, Khoshbouei, The Sigma-1 Receptor as a Regulator of Dopamine Neurotransmission: A Potential Therapeutic Target for Methamphetamine Addiction, Pharmacol. Ther, doi:10.1016/j.pharmthera.2018.01.009
Sanderson, Hisner, Donovan-Banfield, Hartman, Løchen et al., A Molnupiravir-Associated Mutational Signature in Global SARS-CoV-2 Genomes, Nature, doi:10.1038/s41586-023-06649-6
Schafer, Cheng, Xiong, Soloveva, Retterer et al., Repurposing Potential of 1st Generation H1-Specific Antihistamines as Anti-Filovirus Therapeutics, Antiviral Res, doi:10.1016/j.antiviral.2018.07.003
Seminerio, Robson, Abdelazeem, Mesangeau, Jamalapuram et al., Synthesis and Pharmacological Characterization of a Novel Sigma Receptor Ligand with Improved Metabolic Stability and Antagonistic Effects Against Methamphetamine, AAPS J, doi:10.1208/s12248-011-9311-8
Serrano, Kochergina, Albors, Diaz, Oroval et al., Liposomal Lactoferrin as Potential Preventative and Cure for COVID-19, Int. J. Res. Health Sci, doi:10.5530/ijrhs.8.1.3
Sicari, Zabbo, Diphenhydramine, None
Towler, Staker, Prasad, Menon, Tang et al., ACE2 X-Ray Structures Reveal a Large Hinge-Bending Motion Important for Inhibitor Binding and Catalysis, J. Biol. Chem, doi:10.1074/jbc.M311191200
Van Der Strate, Beljaars, Molema, Harmsen, Meijer, Antiviral Activities of Lactoferrin, Antiviral Res, doi:10.1016/S0166-3542(01)00195-4
Vaughan, Omicron Emerges, New Sci, doi:10.1016/S0262-4079(21)02140-0
Wakabayashi, Yamauchi, Takase, Lactoferrin Research, Technology and Applications, Int. Dairy J, doi:10.1016/j.idairyj.2006.06.013
Yan, Zhang, Li, Xia, Guo et al., Structural Basis for the Recognition of SARS-CoV-2 by Full-Length Human ACE2, Science, doi:10.1126/science.abb2762
Yan, Zhang, Zhou, Li, Huang et al., A Web Server for Protein-Protein and Protein-DNA/RNA Docking Based on a Hybrid Strategy, Nucleic Acids Res, doi:10.1093/nar/gkx407
Yang, Wang, Sun, The Roles of Intracellular Chaperone Proteins, Sigma Receptors, in Parkinson's Disease (PD) and Major Depressive Disorder (MDD), Front. Pharmacol, doi:10.3389/fphar.2019.00528
Zheng, Wong, Li, Verma, Ortiz et al., COVID-19 Treatments and Pathogenesis Including Anosmia in K18-hACE2 Mice, Nature, doi:10.1038/s41586-020-2943-z
Zhou, Gammeltoft, Ryberg, Pham, Tjørnelund et al., Nirmatrelvir-Resistant SARS-CoV-2 Variants with High Fitness in an Infectious Cell Culture System, Sci. Adv, doi:10.1126/sciadv.add7197
DOI record:
{
"DOI": "10.3390/ijms242115718",
"ISSN": [
"1422-0067"
],
"URL": "http://dx.doi.org/10.3390/ijms242115718",
"abstract": "<jats:p>The emergence of lethal coronaviruses follows a periodic pattern which suggests a recurring cycle of outbreaks. It remains uncertain as to when the next lethal coronavirus will emerge, though its eventual emergence appears to be inevitable. New mutations in evolving SARS-CoV-2 variants have provided resistance to current antiviral drugs, monoclonal antibodies, and vaccines, reducing their therapeutic efficacy. This underscores the urgent need to investigate alternative therapeutic approaches. Sigma receptors have been unexpectedly linked to the SARS-CoV-2 life cycle due to the direct antiviral effect of their ligands. Coronavirus-induced cell stress facilitates the formation of an ER-derived complex conducive to its replication. Sigma receptor ligands are believed to prevent the formation of this complex. Repurposing FDA-approved drugs for COVID-19 offers a timely and cost-efficient strategy to find treatments with established safety profiles. Notably, diphenhydramine, a sigma receptor ligand, is thought to counteract the virus by inhibiting the creation of ER-derived replication vesicles. Furthermore, lactoferrin, a well-characterized immunomodulatory protein, has shown antiviral efficacy against SARS-CoV-2 both in laboratory settings and in living organisms. In the present study, we aimed to explore the impact of sigma receptor ligands on SARS-CoV-2-induced mortality in ACE2-transgenic mice. We assessed the effects of an investigational antiviral drug combination comprising a sigma receptor ligand and an immunomodulatory protein. Mice treated with sigma-2 receptor ligands or diphenhydramine and lactoferrin exhibited improved survival rates and rapid rebound in mass following the SARS-CoV-2 challenge compared to mock-treated animals. Clinical translation of these findings may support the discovery of new treatment and research strategies for SARS-CoV-2.</jats:p>",
"alternative-id": [
"ijms242115718"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2242-5099",
"affiliation": [
{
"name": "Department of Pathology, Immunology and Laboratory Medicine, College of Medicine, University of Florida, Gainesville, FL 32610, USA"
}
],
"authenticated-orcid": false,
"family": "Berkowitz",
"given": "Reed L.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Spatial Epidemiology and Ecology Research Laboratory, Department of Geography, College of Liberal Arts and Sciences, University of Florida, Gainesville, FL 32611, USA"
},
{
"name": "Emerging Pathogens Institute, University of Florida, Gainesville, FL 32601, USA"
}
],
"family": "Bluhm",
"given": "Andrew P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology, Immunology and Laboratory Medicine, College of Medicine, University of Florida, Gainesville, FL 32610, USA"
}
],
"family": "Knox",
"given": "Glenn W.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8695-2915",
"affiliation": [
{
"name": "Department of Medicinal Chemistry, College of Pharmacy, University of Florida, Gainesville, FL 32610, USA"
},
{
"name": "Translational Drug Development Core, Clinical and Translational Sciences Institute, University of Florida, Gainesville, FL 32610, USA"
}
],
"authenticated-orcid": false,
"family": "McCurdy",
"given": "Christopher R.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4696-875X",
"affiliation": [
{
"name": "Department of Pathology, Immunology and Laboratory Medicine, College of Medicine, University of Florida, Gainesville, FL 32610, USA"
}
],
"authenticated-orcid": false,
"family": "Ostrov",
"given": "David A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5285-0432",
"affiliation": [
{
"name": "Emerging Pathogens Institute, University of Florida, Gainesville, FL 32601, USA"
},
{
"name": "School of Life Sciences, University of Hawaiʻi at Mānoa, Honolulu, HI 96822, USA"
}
],
"authenticated-orcid": false,
"family": "Norris",
"given": "Michael H.",
"sequence": "additional"
}
],
"container-title": "International Journal of Molecular Sciences",
"container-title-short": "IJMS",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
10,
29
]
],
"date-time": "2023-10-29T09:01:08Z",
"timestamp": 1698570068000
},
"deposited": {
"date-parts": [
[
2023,
10,
29
]
],
"date-time": "2023-10-29T09:11:22Z",
"timestamp": 1698570682000
},
"funder": [
{
"name": "University of Florida College of Pharmacy- Pharmacy Recurring Opportunity Seed Program for Education and Research (PROSPER) Excellence Award"
}
],
"indexed": {
"date-parts": [
[
2023,
10,
30
]
],
"date-time": "2023-10-30T09:53:29Z",
"timestamp": 1698659609178
},
"is-referenced-by-count": 0,
"issue": "21",
"issued": {
"date-parts": [
[
2023,
10,
29
]
]
},
"journal-issue": {
"issue": "21",
"published-online": {
"date-parts": [
[
2023,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
29
]
],
"date-time": "2023-10-29T00:00:00Z",
"timestamp": 1698537600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1422-0067/24/21/15718/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "15718",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
10,
29
]
]
},
"published-online": {
"date-parts": [
[
2023,
10,
29
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.3389/fphar.2019.00528",
"article-title": "The Roles of Intracellular Chaperone Proteins, Sigma Receptors, in Parkinson’s Disease (PD) and Major Depressive Disorder (MDD)",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "528",
"journal-title": "Front. Pharmacol.",
"key": "ref_1",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.3389/fnins.2019.00733",
"article-title": "The Sigma-1 Receptor in Cellular Stress Signaling",
"author": "Hayashi",
"doi-asserted-by": "crossref",
"first-page": "733",
"journal-title": "Front. Neurosci.",
"key": "ref_2",
"volume": "13",
"year": "2019"
},
{
"DOI": "10.1007/164_2017_9",
"article-title": "Sigma-1 Receptor and Pain",
"author": "Merlos",
"doi-asserted-by": "crossref",
"first-page": "131",
"journal-title": "Handb. Exp. Pharmacol.",
"key": "ref_3",
"volume": "244",
"year": "2017"
},
{
"DOI": "10.1016/j.pharmthera.2018.01.009",
"article-title": "The Sigma-1 Receptor as a Regulator of Dopamine Neurotransmission: A Potential Therapeutic Target for Methamphetamine Addiction",
"author": "Sambo",
"doi-asserted-by": "crossref",
"first-page": "152",
"journal-title": "Pharmacol. Ther.",
"key": "ref_4",
"volume": "186",
"year": "2018"
},
{
"DOI": "10.1016/j.virusres.2014.09.016",
"article-title": "Coronavirus-Induced ER Stress Response and Its Involvement in Regulation of Coronavirus–Host Interactions",
"author": "Fung",
"doi-asserted-by": "crossref",
"first-page": "110",
"journal-title": "Virus Res.",
"key": "ref_5",
"volume": "194",
"year": "2014"
},
{
"DOI": "10.3390/pathogens10111514",
"doi-asserted-by": "crossref",
"key": "ref_6",
"unstructured": "Ostrov, D.A., Bluhm, A.P., Li, D., Khan, J.Q., Rohamare, M., Rajamanickam, K., Bhanumathy, K.K., Lew, J., Falzarano, D., and Vizeacoumar, F.J. (2021). Highly Specific Sigma Receptor Ligands Exhibit Anti-Viral Properties in SARS-CoV-2 Infected Cells. Pathogens, 10."
},
{
"DOI": "10.1016/j.bbrc.2020.11.095",
"article-title": "Identification of Antiviral Antihistamines for COVID-19 Repurposing",
"author": "Reznikov",
"doi-asserted-by": "crossref",
"first-page": "173",
"journal-title": "Biochem. Biophys. Res. Commun.",
"key": "ref_7",
"volume": "538",
"year": "2021"
},
{
"DOI": "10.1073/pnas.2105815118",
"article-title": "Morphological Cell Profiling of SARS-CoV-2 Infection Identifies Drug Repurposing Candidates for COVID-19",
"author": "Mirabelli",
"doi-asserted-by": "crossref",
"first-page": "e2105815118",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_8",
"volume": "118",
"year": "2021"
},
{
"DOI": "10.1080/22221751.2021.1888660",
"article-title": "The in Vitro Antiviral Activity of Lactoferrin against Common Human Coronaviruses and SARS-CoV-2 Is Mediated by Targeting the Heparan Sulfate Co-Receptor",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "317",
"journal-title": "Emerg. Microbes Infect.",
"key": "ref_9",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2943-z",
"article-title": "COVID-19 Treatments and Pathogenesis Including Anosmia in K18-hACE2 Mice",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "603",
"journal-title": "Nature",
"key": "ref_10",
"volume": "589",
"year": "2021"
},
{
"DOI": "10.1080/22221751.2020.1838955",
"article-title": "Comparison of Transgenic and Adenovirus hACE2 Mouse Models for SARS-CoV-2 Infection",
"author": "Rathnasinghe",
"doi-asserted-by": "crossref",
"first-page": "2433",
"journal-title": "Emerg. Microbes Infect.",
"key": "ref_11",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.4000/cybergeo.12803",
"doi-asserted-by": "crossref",
"key": "ref_12",
"unstructured": "Banos, A., and Lacasa, J. (2007). Spatio-Temporal Exploration of SARS Epidemic. Cybergeo Eur. J. Geogr., Systems, Modelling, Geostatistics, document 408."
},
{
"key": "ref_13",
"unstructured": "(2023, February 19). CSR MERS Outbreaks. Available online: http://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html."
},
{
"DOI": "10.1016/S1473-3099(20)30484-9",
"article-title": "Comparing SARS-CoV-2 with SARS-CoV and Influenza Pandemics",
"author": "Petersen",
"doi-asserted-by": "crossref",
"first-page": "e238",
"journal-title": "Lancet Infect. Dis.",
"key": "ref_14",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.tim.2021.02.007",
"article-title": "Live and Wet Markets: Food Access versus the Risk of Disease Emergence",
"author": "Naguib",
"doi-asserted-by": "crossref",
"first-page": "573",
"journal-title": "Trends Microbiol.",
"key": "ref_15",
"volume": "29",
"year": "2021"
},
{
"DOI": "10.1016/j.puhip.2022.100350",
"article-title": "Differences in Case-Fatality-Rate of Emerging SARS-CoV-2 Variants",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "100350",
"journal-title": "Public Health Pract.",
"key": "ref_16",
"volume": "5",
"year": "2023"
},
{
"key": "ref_17",
"unstructured": "U.S. Food and Drug Administration (2023, March 21). Coronavirus (COVID-19) Update: FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose, Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use."
},
{
"article-title": "Omicron Emerges",
"author": "Vaughan",
"first-page": "7",
"journal-title": "New Sci.",
"key": "ref_18",
"volume": "252",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2206449",
"article-title": "Rebound of SARS-CoV-2 Infection after Nirmatrelvir–Ritonavir Treatment",
"author": "Charness",
"doi-asserted-by": "crossref",
"first-page": "1045",
"journal-title": "N. Engl. J. Med.",
"key": "ref_19",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1101/2022.06.07.495116",
"doi-asserted-by": "crossref",
"key": "ref_20",
"unstructured": "Jochmans, D., Liu, C., Donckers, K., Stoycheva, A., Boland, S., Stevens, S.K., De Vita, C., Vanmechelen, B., Maes, P., and Trüeb, B. (2022). The Substitutions L50F, E166A and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance to Nirmatrelvir, Microbiology."
},
{
"DOI": "10.1126/sciadv.add7197",
"article-title": "Nirmatrelvir-Resistant SARS-CoV-2 Variants with High Fitness in an Infectious Cell Culture System",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "eadd7197",
"journal-title": "Sci. Adv.",
"key": "ref_21",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1101/2022.06.28.497978",
"doi-asserted-by": "crossref",
"key": "ref_22",
"unstructured": "Hu, Y., Lewandowski, E.M., Tan, H., Zhang, X., Morgan, R.T., Zhang, X., Jacobs, L.M.C., Butler, S.G., Gongora, M.V., and Choy, J. (2022). Naturally Occurring Mutations of SARS-CoV-2 Main Protease Confer Drug Resistance to Nirmatrelvir, Pharmacology and Toxicology."
},
{
"DOI": "10.1021/acs.chemrev.0c00648",
"article-title": "Drug Design Strategies to Avoid Resistance in Direct-Acting Antivirals and Beyond",
"author": "Matthew",
"doi-asserted-by": "crossref",
"first-page": "3238",
"journal-title": "Chem. Rev.",
"key": "ref_23",
"volume": "121",
"year": "2021"
},
{
"DOI": "10.1038/s41586-023-06649-6",
"doi-asserted-by": "crossref",
"key": "ref_24",
"unstructured": "Sanderson, T., Hisner, R., Donovan-Banfield, I., Hartman, H., Løchen, A., Peacock, T.P., and Ruis, C. (2023). A Molnupiravir-Associated Mutational Signature in Global SARS-CoV-2 Genomes. Nature."
},
{
"DOI": "10.1038/s41594-021-00651-0",
"article-title": "Mechanism of Molnupiravir-Induced SARS-CoV-2 Mutagenesis",
"author": "Kabinger",
"doi-asserted-by": "crossref",
"first-page": "740",
"journal-title": "Nat. Struct. Mol. Biol.",
"key": "ref_25",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1038/d41586-021-03667-0",
"doi-asserted-by": "crossref",
"key": "ref_26",
"unstructured": "Kozlov, M. (2021). Merck’s COVID Pill Loses Its Lustre: What That Means for the Pandemic. Nature."
},
{
"key": "ref_27",
"unstructured": "COVID-19 Treatment Guidelines Panel (2023, September 26). Coronavirus Disease 2019 (COVID-19) Treatment Guidelines, Available online: https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf."
},
{
"DOI": "10.1038/s41586-022-04594-4",
"article-title": "Antibody Evasion Properties of SARS-CoV-2 Omicron Sublineages",
"author": "Iketani",
"doi-asserted-by": "crossref",
"first-page": "553",
"journal-title": "Nature",
"key": "ref_28",
"volume": "604",
"year": "2022"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"article-title": "A SARS-CoV-2 Protein Interaction Map Reveals Targets for Drug Repurposing",
"author": "Gordon",
"doi-asserted-by": "crossref",
"first-page": "459",
"journal-title": "Nature",
"key": "ref_29",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1016/j.virol.2020.12.007",
"article-title": "Coronavirus Infection Induces Progressive Restructuring of the Endoplasmic Reticulum Involving the Formation and Degradation of Double Membrane Vesicles",
"author": "Mihelc",
"doi-asserted-by": "crossref",
"first-page": "9",
"journal-title": "Virology",
"key": "ref_30",
"volume": "556",
"year": "2021"
},
{
"DOI": "10.1038/s41586-022-04835-6",
"article-title": "The Role of NSP6 in the Biogenesis of the SARS-CoV-2 Replication Organelle",
"author": "Ricciardi",
"doi-asserted-by": "crossref",
"first-page": "761",
"journal-title": "Nature",
"key": "ref_31",
"volume": "606",
"year": "2022"
},
{
"DOI": "10.1186/1743-422X-10-213",
"article-title": "Isolation and Genetic Characterization of Human Coronavirus NL63 in Primary Human Renal Proximal Tubular Epithelial Cells Obtained from a Commercial Supplier, and Confirmation of Its Replication in Two Different Types of Human Primary Kidney Cells",
"author": "Lednicky",
"doi-asserted-by": "crossref",
"first-page": "213",
"journal-title": "Virol. J.",
"key": "ref_32",
"volume": "10",
"year": "2013"
},
{
"DOI": "10.1016/j.cgh.2017.02.020",
"article-title": "Real-World Effectiveness and Safety of Oral Combination Antiviral Therapy for Hepatitis C Virus Genotype 4 Infection",
"author": "Crespo",
"doi-asserted-by": "crossref",
"first-page": "945",
"journal-title": "Clin. Gastroenterol. Hepatol.",
"key": "ref_33",
"volume": "15",
"year": "2017"
},
{
"DOI": "10.1111/hiv.12716",
"article-title": "Two-Drug vs. Three-Drug Combinations for HIV-1: Do We Have Enough Data to Make the Switch?",
"author": "Moreno",
"doi-asserted-by": "crossref",
"first-page": "2",
"journal-title": "HIV Med.",
"key": "ref_34",
"volume": "20",
"year": "2019"
},
{
"key": "ref_35",
"unstructured": "Sicari, V., and Zabbo, C.P. (2023). StatPearls, StatPearls Publishing."
},
{
"DOI": "10.1016/j.antiviral.2018.07.003",
"article-title": "Repurposing Potential of 1st Generation H1-Specific Antihistamines as Anti-Filovirus Therapeutics",
"author": "Schafer",
"doi-asserted-by": "crossref",
"first-page": "47",
"journal-title": "Antiviral Res.",
"key": "ref_36",
"volume": "157",
"year": "2018"
},
{
"DOI": "10.1016/j.idairyj.2006.06.013",
"article-title": "Lactoferrin Research, Technology and Applications",
"author": "Wakabayashi",
"doi-asserted-by": "crossref",
"first-page": "1241",
"journal-title": "Int. Dairy J.",
"key": "ref_37",
"volume": "16",
"year": "2006"
},
{
"DOI": "10.3390/jcm10235482",
"doi-asserted-by": "crossref",
"key": "ref_38",
"unstructured": "Presti, S., Manti, S., Parisi, G.F., Papale, M., Barbagallo, I.A., Li Volti, G., and Leonardi, S. (2021). Lactoferrin: Cytokine Modulation and Application in Clinical Practice. J. Clin. Med., 10."
},
{
"key": "ref_39",
"unstructured": "U.S. Food and Drug Administration U.S. FDA (2023, March 22). GRN 000465 [Cow’s Milk-Derived Lactoferrin], Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=465."
},
{
"DOI": "10.1016/S0166-3542(01)00195-4",
"article-title": "Antiviral Activities of Lactoferrin",
"author": "Beljaars",
"doi-asserted-by": "crossref",
"first-page": "225",
"journal-title": "Antiviral Res.",
"key": "ref_40",
"volume": "52",
"year": "2001"
},
{
"DOI": "10.1111/j.1349-7006.2002.tb02484.x",
"article-title": "Dose-Response Trial of Lactoferrin in Patients with Chronic Hepatitis C",
"author": "Okada",
"doi-asserted-by": "crossref",
"first-page": "1063",
"journal-title": "Jpn. J. Cancer Res.",
"key": "ref_41",
"volume": "93",
"year": "2002"
},
{
"DOI": "10.1158/1940-6207.CAPR-08-0208",
"article-title": "Effect of Orally Administered Bovine Lactoferrin on the Growth of Adenomatous Colorectal Polyps in a Randomized, Placebo-Controlled Clinical Trial",
"author": "Kozu",
"doi-asserted-by": "crossref",
"first-page": "975",
"journal-title": "Cancer Prev. Res.",
"key": "ref_42",
"volume": "2",
"year": "2009"
},
{
"DOI": "10.3390/nu15051285",
"doi-asserted-by": "crossref",
"key": "ref_43",
"unstructured": "Matino, E., Tavella, E., Rizzi, M., Avanzi, G.C., Azzolina, D., Battaglia, A., Becco, P., Bellan, M., Bertinieri, G., and Bertoletti, M. (2023). Effect of Lactoferrin on Clinical Outcomes of Hospitalized Patients with COVID-19: The LAC Randomized Clinical Trial. Nutrients, 15."
},
{
"DOI": "10.1007/s10534-022-00477-3",
"article-title": "Bovine Lactoferrin for the Prevention of COVID-19 Infection in Health Care Personnel: A Double-Blinded Randomized Clinical Trial (LF-COVID)",
"author": "Navarro",
"doi-asserted-by": "crossref",
"first-page": "463",
"journal-title": "BioMetals",
"key": "ref_44",
"volume": "36",
"year": "2023"
},
{
"DOI": "10.3390/ijerph182010985",
"doi-asserted-by": "crossref",
"key": "ref_45",
"unstructured": "Campione, E., Lanna, C., Cosio, T., Rosa, L., Conte, M.P., Iacovelli, F., Romeo, A., Falconi, M., Del Vecchio, C., and Franchin, E. (2021). Lactoferrin as Antiviral Treatment in COVID-19 Management: Preliminary Evidence. Int. J. Environ. Res. Public. Health, 18."
},
{
"article-title": "Liposomal Lactoferrin as Potential Preventative and Cure for COVID-19",
"author": "Serrano",
"first-page": "08",
"journal-title": "Int. J. Res. Health Sci.",
"key": "ref_46",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.3390/jcm10184276",
"doi-asserted-by": "crossref",
"key": "ref_47",
"unstructured": "Rosa, L., Tripepi, G., Naldi, E., Aimati, M., Santangeli, S., Venditto, F., Caldarelli, M., and Valenti, P. (2021). Ambulatory COVID-19 Patients Treated with Lactoferrin as a Supplementary Antiviral Agent: A Preliminary Study. J. Clin. Med., 10."
},
{
"DOI": "10.3390/ijms23105436",
"doi-asserted-by": "crossref",
"key": "ref_48",
"unstructured": "Piacentini, R., Centi, L., Miotto, M., Milanetti, E., Di Rienzo, L., Pitea, M., Piazza, P., Ruocco, G., Boffi, A., and Parisi, G. (2022). Lactoferrin Inhibition of the Complex Formation between ACE2 Receptor and SARS CoV-2 Recognition Binding Domain. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.3390/jcm10020297",
"doi-asserted-by": "crossref",
"key": "ref_49",
"unstructured": "Cappanera, S., Palumbo, M., Kwan, S.H., Priante, G., Martella, L.A., Saraca, L.M., Sicari, F., Vernelli, C., Di Giuli, C., and Andreani, P. (2021). When Does the Cytokine Storm Begin in COVID-19 Patients? A Quick Score to Recognize It. J. Clin. Med., 10."
},
{
"DOI": "10.1186/s13073-022-01013-1",
"article-title": "Genome-Scale CRISPR Screens Identify Host Factors That Promote Human Coronavirus Infection",
"author": "Grodzki",
"doi-asserted-by": "crossref",
"first-page": "10",
"journal-title": "Genome Med.",
"key": "ref_50",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2019.00678",
"article-title": "Characterization of Sigma 1 Receptor Antagonist CM-304 and Its Analog, AZ-66: Novel Therapeutics Against Allodynia and Induced Pain",
"author": "Cirino",
"doi-asserted-by": "crossref",
"first-page": "678",
"journal-title": "Front. Pharmacol.",
"key": "ref_51",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1021/jm300371c",
"article-title": "New Positron Emission Tomography (PET) Radioligand for Imaging σ-1 Receptors in Living Subjects",
"author": "James",
"doi-asserted-by": "crossref",
"first-page": "8272",
"journal-title": "J. Med. Chem.",
"key": "ref_52",
"volume": "55",
"year": "2012"
},
{
"DOI": "10.1208/s12248-011-9311-8",
"article-title": "Synthesis and Pharmacological Characterization of a Novel Sigma Receptor Ligand with Improved Metabolic Stability and Antagonistic Effects Against Methamphetamine",
"author": "Seminerio",
"doi-asserted-by": "crossref",
"first-page": "43",
"journal-title": "AAPS J.",
"key": "ref_53",
"volume": "14",
"year": "2012"
},
{
"DOI": "10.1208/s12248-020-00472-x",
"article-title": "Discovery of a Highly Selective Sigma-2 Receptor Ligand, 1-(4-(6,7-Dimethoxy-3,4-Dihydroisoquinolin-2(1H)-Yl)Butyl)-3-Methyl-1H-Benzo[d]Imidazol-2(3H)-One (CM398), with Drug-Like Properties and Antinociceptive Effects In Vivo",
"author": "Intagliata",
"doi-asserted-by": "crossref",
"first-page": "94",
"journal-title": "AAPS J.",
"key": "ref_54",
"volume": "22",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-19891-7",
"article-title": "Lethality of SARS-CoV-2 Infection in K18 Human Angiotensin-Converting Enzyme 2 Transgenic Mice",
"author": "Oladunni",
"doi-asserted-by": "crossref",
"first-page": "6122",
"journal-title": "Nat. Commun.",
"key": "ref_55",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1126/science.abb2762",
"article-title": "Structural Basis for the Recognition of SARS-CoV-2 by Full-Length Human ACE2",
"author": "Yan",
"doi-asserted-by": "crossref",
"first-page": "1444",
"journal-title": "Science",
"key": "ref_56",
"volume": "367",
"year": "2020"
},
{
"DOI": "10.1093/nar/gkx407",
"article-title": "HDOCK: A Web Server for Protein–Protein and Protein–DNA/RNA Docking Based on a Hybrid Strategy",
"author": "Yan",
"doi-asserted-by": "crossref",
"first-page": "W365",
"journal-title": "Nucleic Acids Res.",
"key": "ref_57",
"volume": "45",
"year": "2017"
},
{
"DOI": "10.1074/jbc.M311191200",
"article-title": "ACE2 X-Ray Structures Reveal a Large Hinge-Bending Motion Important for Inhibitor Binding and Catalysis",
"author": "Towler",
"doi-asserted-by": "crossref",
"first-page": "17996",
"journal-title": "J. Biol. Chem.",
"key": "ref_58",
"volume": "279",
"year": "2004"
},
{
"DOI": "10.1006/jmbi.1997.1386",
"article-title": "Three-Dimensional Structure of Diferric Bovine Lactoferrin at 2.8 Å Resolution",
"author": "Moore",
"doi-asserted-by": "crossref",
"first-page": "222",
"journal-title": "J. Mol. Biol.",
"key": "ref_59",
"volume": "274",
"year": "1997"
},
{
"DOI": "10.1038/nrd.2017.162",
"article-title": "Host-Directed Therapies for Bacterial and Viral Infections",
"author": "Kaufmann",
"doi-asserted-by": "crossref",
"first-page": "35",
"journal-title": "Nat. Rev. Drug Discov.",
"key": "ref_60",
"volume": "17",
"year": "2018"
}
],
"reference-count": 60,
"references-count": 60,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1422-0067/24/21/15718"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Inorganic Chemistry",
"Organic Chemistry",
"Physical and Theoretical Chemistry",
"Computer Science Applications",
"Spectroscopy",
"Molecular Biology",
"General Medicine",
"Catalysis"
],
"subtitle": [],
"title": "Sigma Receptor Ligands Prevent COVID Mortality In Vivo: Implications for Future Therapeutics",
"type": "journal-article",
"volume": "24"
}