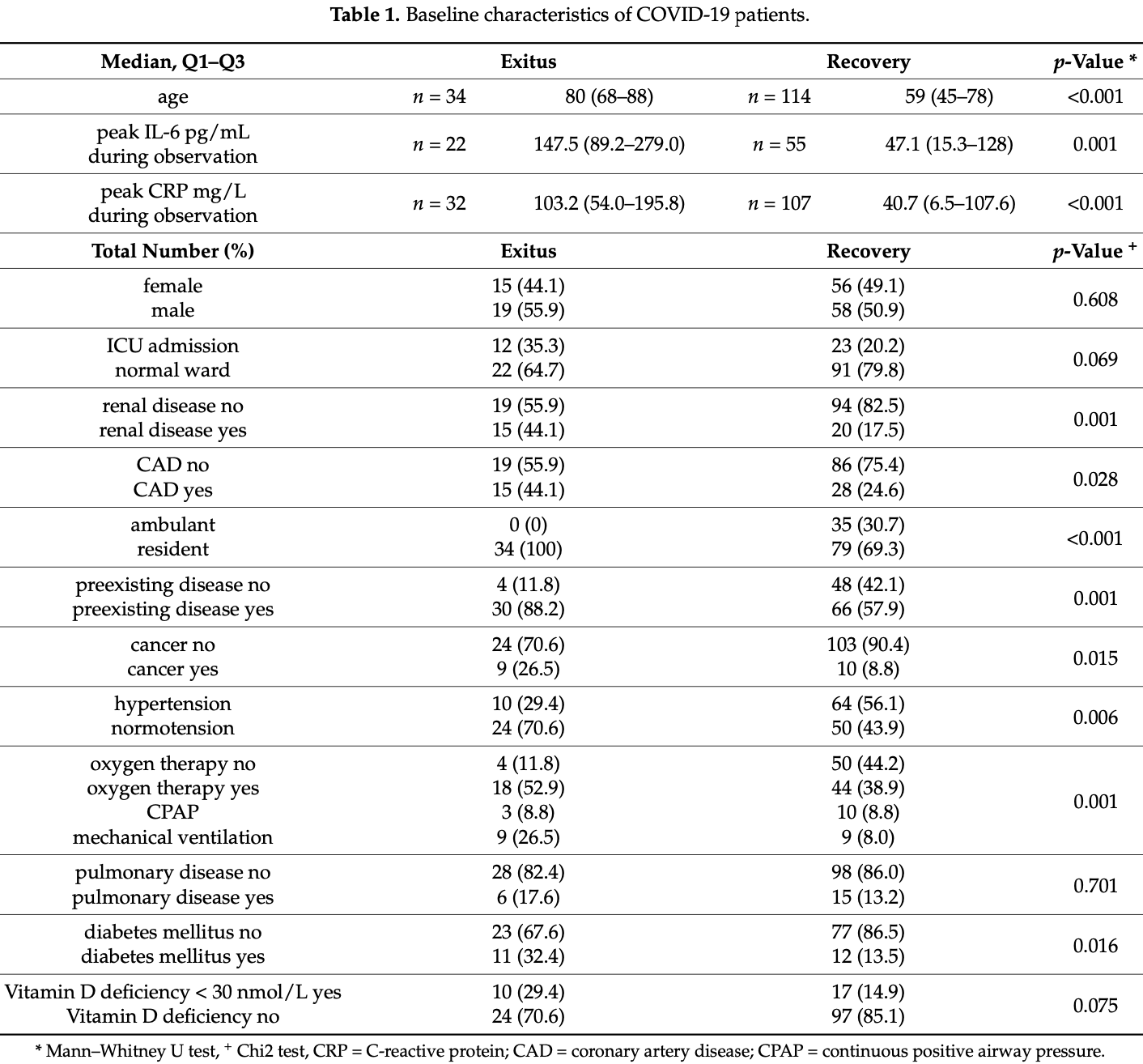
Vitamin D Metabolites and Clinical Outcome in Hospitalized COVID-19 Patients
et al., Nutrients, doi:10.3390/nu13072129, Jun 2021
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 148 patients in Austria, showing no statistically significant differences in vitamin D levels and metabolites for mortality or respiratory support.
This is the 75th of 228 COVID-19 sufficiency studies for vitamin D, which collectively show higher levels reduce risk with p<0.0000000001.
|
risk of death, 46.4% lower, RR 0.54, p = 0.08, high D levels 24 of 121 (19.8%), low D levels 10 of 27 (37.0%), NNT 5.8, >30nmol/L.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Zelzer et al., 22 Jun 2021, retrospective, Austria, peer-reviewed, 7 authors.
Vitamin D Metabolites and Clinical Outcome in Hospitalized COVID-19 Patients
Nutrients, doi:10.3390/nu13072129
Metabolites and Clinical Outcome in Hospitalized COVID-19 Patients.
Conflicts of Interest: The authors declare no conflict of interest.
References
Abrishami, Dalili, Torbati, Asgari, Arab-Ahmadi et al., Possible association of vitamin D status with lung involvement and outcome in patients with COVID-19: A retrospective study, Eur. J. Nutr, doi:10.1007/s00394-020-02411-0
Akbar, Wibowo, Pranata, Setiabudiawan, Low Serum 25-hydroxyvitamin D (Vitamin D) Level Is Associated With Susceptibility to COVID-19, Severity, and Mortality: A Systematic Review and Meta-Analysis, Front. Nutr
Al-Daghri, Amer, Alotaibi, Aldisi, Enani et al., Vitamin D status of Arab Gulf residents screened for SARS-CoV-2 and its association with COVID-19 infection: A multi-centre case-control study, J. Transl. Med, doi:10.1186/s12967-021-02838-x
Alguwaihes, Sabico, Hasanato, Al-Sofiani, Megdad et al., Severe vitamin D deficiency is not related to SARS-CoV-2 infection but may increase mortality risk in hospitalized adults: A retrospective case-control study in an Arab Gulf country, Aging Clin. Exp. Res
Ali, Role of vitamin D in preventing of COVID-19 infection, progression and severity, J. Infect. Public Health
Amrein, Scherkl, Hoffmann, Neuwersch-Sommeregger, Köstenberger et al., deficiency 2.0: An update on the current status worldwide, Eur. J. Clin. Nutr, doi:10.1038/s41430-020-0558-y
Annweiler, Hanotte, Grandin De L'eprevier, Sabatier, Lafaie et al., Vitamin D and survival in COVID-19 patients: A quasi-experimental study, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2020.105771
Baeke, Gysemans, Korf, Mathieu, Vitamin D insufficiency: Implications for the immune system, Pediatr. Nephrol, doi:10.1007/s00467-010-1452-y
Baeke, Korf, Overbergh, Van Etten, Verstuyf et al., Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D 3 in the immune system, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2010.03.037
Baeke, Korf, Overbergh, Verstuyf, Thorrez et al., The vitamin D analog, TX527, promotes a human CD4 + CD25 high CD127 low regulatory T cell profile and induces a migratory signature specific for homing to sites of inflammation, J. Immunol, doi:10.4049/jimmunol.1000695
Baktash, Hosack, Patel, Shah, Kandiah et al., Vitamin D status and outcomes for hospitalised older patients with COVID-19, Postgrad Med. J, doi:10.1136/postgradmedj-2020-138712
Beard, Bearden, Striker, Vitamin D and the anti-viral state, J. Clin. Virol, doi:10.1016/j.jcv.2010.12.006
Bennouar, Cherif, Kessira, Bennouar, Abdi, Vitamin D Deficiency and Low Serum Calcium as Predictors of Poor Prognosis in Patients with Severe COVID-19, J. Am. Coll. Nutr, doi:10.1080/07315724.2020.1856013
Bikle, Vitamin D metabolism, mechanism of action, and clinical applications, Chem. Biol, doi:10.1016/j.chembiol.2013.12.016
Brenner, Vitamin D Supplementation to Prevent COVID-19 Infections and Deaths-Accumulating Evidence from Epidemiological and Intervention Studies Calls for Immediate Action, Nutrients, doi:10.3390/nu13020411
Buonpane, Therapeutic drug monitoring of cyclosporine (CSA), Conn. Med
Cavalier, Fraser, Bhattoa, Heijboer, Makris et al., Analytical Performance Specifications for 25-Hydroxyvitamin D Examinations, Nutrients, doi:10.3390/nu13020431
Cavalier, Huyghebaert, Rousselle, Bekaert, Kovacs et al., Simultaneous measurement of 25(OH)-vitamin D and 24, 25(OH)2-vitamin D to define cut-offs for CYP24A1 mutation and vitamin D deficiency in a population of 1200 young subjects, Clin. Chem. Lab Med, doi:10.1515/cclm-2019-0996
Cereda, Bogliolo, Lobascio, Barichella, Zecchinelli et al., Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy, Italy, Nutrition
Chang, Cha, Lee, Seo, Kweon, 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis, PLoS ONE, doi:10.1371/annotation/6da7b65e-dda2-467d-bcb3-82d5669f6bc6
Charoenngam, Holick, Immunologic Effects of Vitamin D on Human Health and Disease, Nutrients
Coldwell, Trafford, Makin, Varley, Specific mass fragmentographic assay for 25, 26-dihydroxyvitamin D in human plasma using a deuterated internal standard, J. Chromatogr, doi:10.1016/0378-4347(85)80100-6
De Haan, Groeneveld, De Geus, Egal, Struijs, Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: Systematic review and meta-analysis, Crit. Care, doi:10.1186/s13054-014-0660-4
Deluca, Suda, Schnoes, Tanaka, Holick, 26-dihydroxycholecalciferol, a metabolite of vitamin D3 with intestinal calcium transport activity, Biochemistry, doi:10.1021/bi00826a022
Ebadi, Montano-Loza, Perspective: Improving vitamin D status in the management of COVID-19, Eur. J. Clin. Nutr, doi:10.1038/s41430-020-0661-0
Enko, Kriegshauser, Stolba, Worf, Halwachs-Baumann, Method evaluation study of a new generation of vitamin D assays, Biochem. Med, doi:10.11613/BM.2015.020
Farrell, Soldo, Williams, Herrmann, 25-Hydroxyvitamin D testing: Challenging the performance of current automated immunoassays, Clin. Chem. Lab Med, doi:10.1515/cclm-2012-0522
Gimenez, Sanz, Maron, Ferder, Manucha, Vitamin D-RAAS Connection: An Integrative Standpoint into Cardiovascular and Neuroinflammatory Disorders, Curr. Protein Pept. Sci, doi:10.2174/1389203721666200606220719
Gombart, Pierre, Maggini, A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection, Nutrients, doi:10.3390/nu12010236
Grant, Lahore, Mcdonnell, Baggerly, French et al., Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths, Nutrients, doi:10.3390/nu12040988
Hernández, Nan, Fernandez-Ayala, García-Unzueta, Hernández-Hernández et al., Vitamin D Status in Hospitalized Patients with SARS-CoV-2
Holick, The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention, Rev. Endocr. Metab. Disord, doi:10.1007/s11154-017-9424-1
Holick, Vitamin, Deficiency, None, N. Engl. J. Med, doi:10.1056/NEJMra070553
Im, Je, Baek, Chung, Kwon et al., Nutritional status of patients with COVID-19, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.08.018
Jain, Chaurasia, Sengar, Singh, Mahor et al., Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers, Sci. Rep, doi:10.1038/s41598-020-77093-z
Joshi, Pantalena, Liu, Gaffen, Liu et al., 1,25-dihydroxyvitamin D 3 ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A, Mol. Cell. Biol, doi:10.1128/MCB.05020-11
Katz, Yue, Xue, Increased risk for COVID-19 in patients with vitamin D deficiency, Nutrition, doi:10.1016/j.nut.2020.111106
Leaf, Ginde, Vitamin D3 to Treat COVID-19: Different Disease, Same Answer, JAMA, doi:10.1001/jama.2020.26850
Livingston, Plant, Dunmore, Hartland, Jones et al., Detectable respiratory SARS-CoV-2 RNA is associated with low vitamin D levels and high social deprivation, Int. J. Clin. Pract, doi:10.1111/ijcp.14166
Loeffelholz, Alland, Butler-Wu, Pandey, Perno et al., Multicenter Evaluation of the Cepheid Xpert Xpress SARS-CoV-2 Test, J. Clin. Microbiol, doi:10.1128/JCM.00926-20
Luo, Liao, Shen, Li, Cheng, Vitamin D Deficiency Is Associated with COVID-19 Incidence and Disease Severity in Chinese People
Maghbooli, Sahraian, Ebrahimi, Pazoki, Kafan et al., Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection, PLoS ONE, doi:10.1371/journal.pone.0239799
Martineau, Jolliffe, Hooper, Greenberg, Aloia et al., Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data, BMJ, doi:10.1136/bmj.i6583
Murai, Fernandes, Sales, Pinto, Goessler et al., Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.26848
Pairo-Castineira, Clohisey, Klaric, Bretherick, Rawlik et al., Genetic mechanisms of critical illness in COVID-19, Nature, doi:10.1038/s41586-020-03065-y
Penna, Amuchastegui, Cossetti, Aquilano, Mariani et al., Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol, J. Immunol
Prietl, Treiber, Pieber, Amrein, Vitamin D and immune function, Nutrients, doi:10.3390/nu5072502
Rahme, Al-Shaar, Singh, Baddoura, Halaby et al., Limitations of platform assays to measure serum 25OHD level impact on guidelines and practice decision making, Metabolism, doi:10.1016/j.metabol.2018.09.003
Szeto, Zucker, Lasota, Rubin, Walker et al., Vitamin D Status and COVID-19 Clinical Outcomes in Hospitalized Patients, Endocr. Res, doi:10.1080/07435800.2020.1867162
Thickett, Moromizato, Litonjua, Amrein, Quraishi et al., Association between prehospital vitamin D status and incident acute respiratory failure in critically ill patients: A retrospective cohort study, BMJ Open Respir. Res, doi:10.1136/bmjresp-2014-000074
Wolters, Grünberg, Huber, Kessler, Prüller et al., European multicenter evaluation of Xpert ® Xpress SARS-CoV-2/Flu/RSV test, J. Med. Virol, doi:10.1002/jmv.27111
Yisak, Ewunetei, Kefale, Mamuye, Teshome et al., Effects of Vitamin D on COVID-19 Infection and Prognosis: A Systematic Review, Risk Manag. Healthc. Policy, doi:10.2147/RMHP.S291584
Zelzer, Hofer, Meinitzer, Fritz-Petrin, Simstich et al., Association of vitamin D metabolites with cognitive function and brain atrophy in elderly individuals-The Austrian stroke prevention study, Aging, doi:10.18632/aging.202930
Zelzer, Meinitzer, Enko, Simstich, Le Goff et al., Simultaneous determination of 24, 25-and 25, 26-dihydroxyvitamin D 3 in serum samples with liquid-chromatography mass spectrometry-A useful tool for the assessment of vitamin D metabolism, J. Chromatogr. B Anal. Technol. Biomed. Life Sci, doi:10.1016/j.jchromb.2020.122394
DOI record:
{
"DOI": "10.3390/nu13072129",
"ISSN": [
"2072-6643"
],
"URL": "http://dx.doi.org/10.3390/nu13072129",
"abstract": "<jats:p>(1) Background: Vitamin D, a well-established regulator of calcium and phosphate metabolism, also has immune-modulatory functions. An uncontrolled immune response and cytokine storm are tightly linked to fatal courses of COVID-19. The present retrospective study aimed to inves-tigate vitamin D status markers and vitamin D degradation products in a mixed cohort of 148 hospitalized COVID-19 patients with various clinical courses of COVID-19. (2) Methods: The serum concentrations of 25(OH)D3, 25(OH)D2, 24,25(OH)2D3, and 25,26(OH)2D3 were determined by a validated liquid-chromatography tandem mass-spectrometry method in leftover serum samples from 148 COVID-19 patients that were admitted to the University Hospital of the Medical Uni-versity of Graz between April and November 2020. Anthropometric and clinical data, as well as outcomes were obtained from the laboratory and hospital information systems. (3) Results: From the 148 patients, 34 (23%) died within 30 days after admission. The frequency of fatal outcomes did not differ between males and females. Non-survivors were significantly older than survivors, had higher peak concentrations of IL-6 and CRP, and required mechanical ventilation more frequently. The serum concentrations of all vitamin D metabolites and the vitamin D metabolite ratio (VMR) did not differ significantly between survivors and non-survivors. Additionally, the need for res-piratory support was unrelated to the serum concentrations of 25(OH)D vitamin D and the two vitamin D catabolites, as well as the VMR. (4) Conclusion: The present results do not support a relevant role of vitamin D for the course and outcome of COVID-19.</jats:p>",
"alternative-id": [
"nu13072129"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8653-0779",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zelzer",
"given": "Sieglinde",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-3388-0089",
"affiliation": [],
"authenticated-orcid": false,
"family": "Prüller",
"given": "Florian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Curcic",
"given": "Pero",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sloup",
"given": "Zdenka",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8355-3617",
"affiliation": [],
"authenticated-orcid": false,
"family": "Holter",
"given": "Magdalena",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3559-9899",
"affiliation": [],
"authenticated-orcid": false,
"family": "Herrmann",
"given": "Markus",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4067-247X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mangge",
"given": "Harald",
"sequence": "additional"
}
],
"container-title": "Nutrients",
"container-title-short": "Nutrients",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
6,
23
]
],
"date-time": "2021-06-23T02:10:59Z",
"timestamp": 1624414259000
},
"deposited": {
"date-parts": [
[
2021,
6,
24
]
],
"date-time": "2021-06-24T15:52:34Z",
"timestamp": 1624549954000
},
"indexed": {
"date-parts": [
[
2023,
12,
28
]
],
"date-time": "2023-12-28T13:39:54Z",
"timestamp": 1703770794100
},
"is-referenced-by-count": 16,
"issue": "7",
"issued": {
"date-parts": [
[
2021,
6,
22
]
]
},
"journal-issue": {
"issue": "7",
"published-online": {
"date-parts": [
[
2021,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
6,
22
]
],
"date-time": "2021-06-22T00:00:00Z",
"timestamp": 1624320000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2072-6643/13/7/2129/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "2129",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
6,
22
]
]
},
"published-online": {
"date-parts": [
[
2021,
6,
22
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1007/s11154-017-9424-1",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1016/j.chembiol.2013.12.016",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.3390/nu5072502",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1056/NEJMra070553",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1038/s41586-020-03065-y",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1007/s40520-021-01831-0",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1080/07315724.2020.1856013",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.3390/nu12072097",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.4049/jimmunol.177.12.8504",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1128/MCB.05020-11",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1371/annotation/6da7b65e-dda2-467d-bcb3-82d5669f6bc6",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1136/bmj.i6583",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.3390/nu12010236",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.4049/jimmunol.1000695",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1016/j.jsbmb.2010.03.037",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1007/s00467-010-1452-y",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1186/s13054-014-0660-4",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1136/bmjresp-2014-000074",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1038/s41598-020-77093-z",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.2147/RMHP.S291584",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1016/j.jcv.2010.12.006",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1016/j.jsbmb.2020.105771",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.3390/nu12040988",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.2174/1389203721666200606220719",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1016/j.nut.2020.111055",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1016/j.jiph.2020.06.021",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1515/cclm-2012-0522",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.11613/BM.2015.020",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.3390/nu13020431",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1515/cclm-2019-0996",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.1021/bi00826a022",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1016/0378-4347(85)80100-6",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1016/j.jchromb.2020.122394",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1128/JCM.00926-20",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1002/jmv.27111",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1111/ijcp.14166",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.3390/nu13020411",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1080/07435800.2020.1867162",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1136/postgradmedj-2020-138712",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1210/clinem/dgaa733",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1186/s12967-021-02838-x",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1016/j.ijid.2020.08.018",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1016/j.nut.2020.111106",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1093/jn/nxaa332",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1371/journal.pone.0239799",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1007/s00394-020-02411-0",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.3389/fnut.2021.660420",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.1016/j.metabol.2018.09.003",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"article-title": "Therapeutic drug monitoring of cyclosporine (CSA)",
"author": "Buonpane",
"first-page": "17",
"journal-title": "Conn. Med.",
"key": "ref49",
"volume": "54",
"year": "1990"
},
{
"DOI": "10.1038/s41430-020-0558-y",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.18632/aging.202930",
"doi-asserted-by": "publisher",
"key": "ref51"
},
{
"DOI": "10.1038/s41430-020-0661-0",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.1001/jama.2020.26848",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"DOI": "10.1001/jama.2020.26850",
"doi-asserted-by": "publisher",
"key": "ref54"
}
],
"reference-count": 54,
"references-count": 54,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2072-6643/13/7/2129"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Food Science",
"Nutrition and Dietetics"
],
"subtitle": [],
"title": "Vitamin D Metabolites and Clinical Outcome in Hospitalized COVID-19 Patients",
"type": "journal-article",
"volume": "13"
}
