Evaluation of Antibacterial and Antiviral Drug Effectiveness in COVID-19 Therapy: A Data-Driven Retrospective Approach
et al., Pathophysiology, doi:10.3390/pathophysiology29010009, Mar 2022
Retrospective hospitalized patients in Indonesia, showing lower mortality and shorter hospitalization with favipiravir.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
risk of death, 85.3% lower, OR 0.15, p = 0.05, inverted to make OR<1 favor treatment, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Yulia et al., 7 Mar 2022, retrospective, Indonesia, peer-reviewed, median age 46.0, 10 authors, study period July 2020 - December 2020.
Contact: fauna@staff.ubaya.ac.id (corresponding author), rika_y@staff.ubaya.ac.id, putriayuirma@gmail.com, rudd_apt@yahoo.com, purisafitrihanum@staff.ubaya.ac.id, herwyno@staff.ubaya.ac.id, lestionoapt@gmail.com, deramdani123@gmail.com, abusuquf@yahoo.co.id, kevin.kantono@aut.ac.nz.
Evaluation of Antibacterial and Antiviral Drug Effectiveness in COVID-19 Therapy: A Data-Driven Retrospective Approach
Pathophysiology, doi:10.3390/pathophysiology29010009
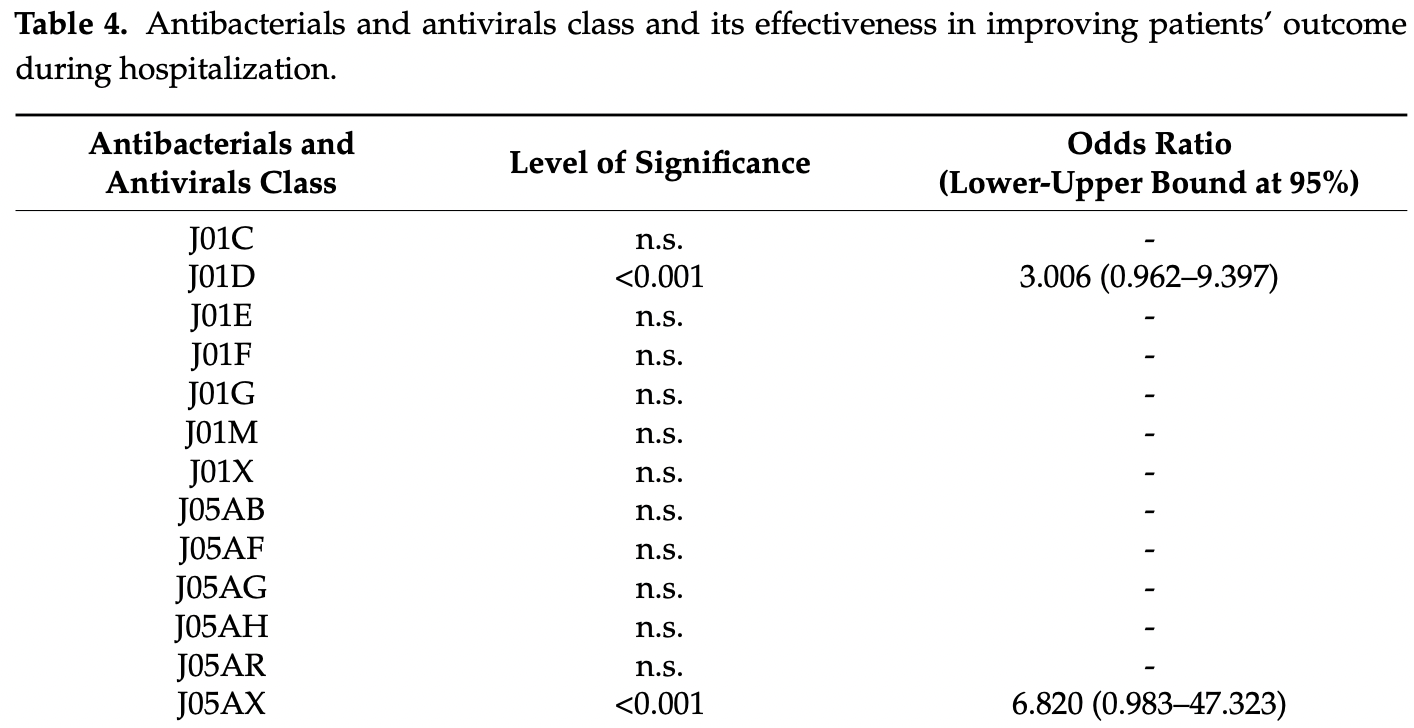
The clinical manifestations associated with COVID-19 disease is mainly due to a dysregulated host response related to the overexpression of inflammatory markers. Until recently, only remdesivir had gained FDA approval for COVID-19 hospitalized patients and there are currently no evidence-based therapeutic options or options for prevention of complications that have been established. Some medical treatments such as antivirals, antibacterials, antithrombotics, antipyretics, corticosteroids, interleukin inhibitors, monoclonal antibodies, convalescent plasma, immunostimulants, and vitamin supplements have been utilized. However, there are limited data to support their effectiveness. Hence, this study was attempted to identify and evaluate the effectiveness of antibacterials and antivirals used for COVID-19 using a retrospective cross-sectional approach based on the medical records of adult patients in four hospitals. The number of antibacterials was calculated in defined daily dose (DDD) per 100 bed-days unit. Both mixed-logit regression and analysis of covariance were used to determine the effectiveness of the aforementioned agents in relation to COVID-19 outcome and patients' length of stay. The model was weighed accordingly and covariates (e.g., age) were considered in the model. Heart disease was found to be the most common pre-existing condition of COVID-19 hospitalized patients in this study. Azithromycin, an antibacterial in the Watch category list, was used extensively (33-65 DDD per 100 bed-days). Oseltamivir, an antiviral approved by the FDA for influenza was the most prescribed antiviral. In addition, favipiravir was found to be a significant factor in improving patients' COVID-19 outcomes and decreasing their length of stay. This study strongly suggests that COVID-19 patients' received polypharmacy for their treatment. However, most of the drugs used did not reach statistical significance in improving the patients' condition or decreasing the length of stay. Further studies to support drug use are needed.
Conflicts of Interest: The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
References
Abubakar, Sani, Godman, Kumar, Islam et al., Systematic review on the therapeutic options for COVID-19: Clinical evidence of drug efficacy and implications, Infect. Drug Resist, doi:10.2147/IDR.S289037
Adebisi, Alaran, Okereke, Oke, Amos et al., COVID-19 and antimicrobial resistance: A review, Infect. Dis. Res. Treat, doi:10.1177/11786337211033870
Aumpan, Vilaichone, Ratana-Amornpin, Teerakapibal, Toochinda et al., Antiviral treatment could not provide clinical benefit in management of mild COVID-19: A retrospective experience from field hospital, J. Infect. Public Health, doi:10.1016/j.jiph.2021.07.019
Baracco, Remdesivir use and hospital length of stay-The paradox of a clinical trial vs real-life use, JAMA
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the Treatment of Covid-19-Final Report, N. Engl. J. Med, doi:10.1056/NEJMoa2007764
Biswas, Rahaman, Biswas, Haque, Ibrahim, Association of sex, age, and comorbidities with mortality in COVID-19 patients: A systematic review and meta-analysis, Intervirology, doi:10.1159/000512592
Bogdanić, Močibob, Vidović, Soldo, Begovać, Azithromycin consumption during the COVID-19 pandemic in Croatia, PLoS ONE, doi:10.1371/journal.pone.0263437
Burhan, Susanto, Nasution, Ginanjar, Pitoyo et al., Tim COVID-19 IDAI
Cai, Yang, Liu, Chen, Shu et al., Experimental treatment with favipiravir for COVID-19: An open-label control study, Engineering, doi:10.1016/j.eng.2020.03.007
Cao, Wang, Wen, Liu, Wang et al., A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2001282
Chedid, Waked, Haddad, Chetata, Saliba et al., Antibiotics in treatment of COVID-19 complications: A review of frequency, indications, and efficacy, J. Infect. Public Health, doi:10.1016/j.jiph.2021.02.001
Chiam, Subedi, Chen, Best, Bianco et al., Hospital length of stay among COVID-19-positive patients, J. Clin. Transl. Res, doi:10.18053/jctres.07.202103.010
Dana, Bannay, Bourst, Ziegler, Losser et al., Obesity and mortality in critically ill COVID-19 patients with respiratory failure, Int. J. Obes, doi:10.1038/s41366-021-00872-9
Egyir, Obeng-Nkrumah, Kyei, COVID-19 pandemic and antimicrobial resistance: Another call to strengthen laboratory diagnostic capacity in Africa, Afr. J. Lab. Med, doi:10.4102/ajlm.v9i1.1302
Ge, Li, Wu, Candido, Wei, Association of pre-existing comorbidities with mortality and disease severity among 167,500 individuals with COVID-19 in Canada: A population-based cohort study, PLoS ONE, doi:10.1371/journal.pone.0258154
Getahun, Smith, Trivedi, Paulin, Balkhy, Tackling antimicrobial resistance in the COVID-19 pandemic, Bull. World Health Organ, doi:10.2471/BLT.20.268573
Ghosh, Bornman, Zafer, Antimicrobial resistance threats in the emerging COVID-19 pandemic: Where do we stand?, J. Infect. Public Health, doi:10.1016/j.jiph.2021.02.011
Giacomelli, Ridolfo, Oreni, Vimercati, Albrecht et al., Consumption of antibiotics at an Italian university hospital during the early months of the COVID-19 pandemic: Were all antibiotic prescriptions appropriate?, Pharmacol. Res, doi:10.1016/j.phrs.2020.105403
Grau, Echeverria-Esnal, Gómez-Zorrilla, Navarreterouco, Masclans et al., Evolution of antimicrobial consumption during the first wave of COVID-19 pandemic, Antibiotics, doi:10.3390/antibiotics10020132
Gupta, Dhar, Swarnakar, Bedi, Chawla, Impact of COVID-19 on (Non-COVID) chronic respiratory disease outcome survey in India (CCROS study), Lung India, doi:10.4103/lungindia.lungindia_965_20
Hartmann-Boyce, Rees, Otunla, Suklan, Schofield et al., Risks of and from SARS-CoV-2 (COVID-19) Infection in People with Asthma. Centre for Evidence-Based Medicine
Hasan, Haider, Stigler, Khan, Mccoy et al., The global Case-Fatality Rate of COVID-19 has been declining since May 2020, Am. J. Trop. Med. Hyg, doi:10.4269/ajtmh.20-1496
Holec, Mandal, Prathipati, Destache, Nucleotide Reverse Transcriptase Inhibitors: A thorough review, present status and future perspective as HIV therapeutics, Curr. HIV Res, doi:10.2174/1570162X15666171120110145
Jang, Seon, Yoon, Park, Lee et al., Comorbidities and factors determining medical expenses and length of stay for admitted COVID-19 patients in Korea, Risk Manag. Healthc. Policy, doi:10.2147/RMHP.S292538
Joshi, Parkar, Ansari, Vora, Talwar et al., Role of favipiravir in the treatment of COVID-19, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.10.069
Kassam, Aghan, Aziz, Mbithe, Hameed et al., Factors associated with mortality among hospitalized adults with COVID-19 pneumonia at a Private Tertiary Hospital in Tanzania: A retrospective cohort study, Int. J. Gen. Med, doi:10.2147/IJGM.S330580
Kim, An, Kim, Hwang, Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: A systematic review and network metaanalysis, PLoS Med, doi:10.1371/journal.pmed.1003501
Kocayigit, Ozmen, Suner, Tomak, Demir et al., Observational study of the effects of favipiravir vs lopinavir/ritonavir on clinical outcomes in critically ill patients with COVID-19, J. Clin. Pharm. Ther, doi:10.1111/jcpt.13305
Limato, Nelwan, Mudia, De Brabander, Guterres et al., A multicentre point prevalence survey of patterns and quality of antibiotic prescribing in Indonesian hospitals, JAC Antimicrob. Resist, doi:10.1093/jacamr/dlab047
Maciorowski, Idrissi, Gupta, Medernach, Burns et al., A review of the preclinical and clinical efficacy of remdesivir, hydroxychloroquine, and lopinavir-ritonavir treatments against COVID-19, SLAS DISCOVERY, doi:10.1177/2472555220958385
Mahendra, Nuchin, Kumar, Shreedhar, Mahesh, Predictors of mortality in patients with severe COVID-19 pneumonia-A retrospective study, Adv. Respir. Med, doi:10.5603/ARM.a2021.0036
Manabe, Kambayashi, Akatsu, Kudo, Favipiravir for the treatment of patients with COVID-19: A systematic review and meta-analysis, BMC Infect. Dis, doi:10.1186/s12879-021-06164-x
Mash, Presence-Vollenhoven, Adeniji, Christoffels, Doubell et al., Evaluation of patient characteristics, management and outcomes for COVID-19 at district hospitals in the Western Cape, South Africa: Descriptive observational study, BMJ Open, doi:10.1136/bmjopen-2020-047016
Mendes, Jara, Mansour, Araújo, Velloso, Asthma and COVID-19: A systematic review, Allergy Asthma Clin. Immunol, doi:10.1186/s13223-020-00509-y
Mustafa, Tolaj, Baftiu, Fejza, Use of antibiotics in COVID-19 ICU patients, J. Infect. Dev. Ctries
Nasrin, Mohaimenul, Chia, Chin, Wen-Shan et al., Obesity and mortality among patients diagnosed with COVID-19: A systematic review and meta-analysis, Front. Med, doi:10.3389/fmed.2021.620044
Olry, Meunier, Délire, Larrey, Horsmans et al., Drug-induced liver injury and COVID-19 infection: The rules remain the same, Drug Saf, doi:10.1007/s40264-020-00954-z
Paul, Pennell, Lemeshow, Standardizing the power of the Hosmer-Lemeshow goodness of fit test in large data sets, Stat. Med, doi:10.1002/sim.5525
Peña, Rascón-Pacheco, Ascencio-Montiel, González-Figueroa, Fernández-Gárate et al., Hypertension, diabetes and obesity, major risk factors for death in patients with COVID-19 in Mexico, Arch. Med. Res, doi:10.1016/j.arcmed.2020.12.002
Pi-Sunyer, The medical risks of obesity, Postgrad. Med, doi:10.3810/pgm.2009.11.2074
Popp, Stegemann, Riemer, Metzendorf, Romero et al., Antibiotics for the treatment of COVID-19, Cochrane Database Syst. Rev, doi:10.1002/14651858.CD015025
Pourhoseingholi, Vahedi, Rahimzadeh, Sample size calculation in medical studies, Gastroenterol. Hepatol. Bed. Bench
Rahmani, Davoudi-Monfared, Nourian, Nabiee, Sadeghi et al., Comparing outcomes of hospitalized patients with moderate and severe COVID-19 following treatment with hydroxychloroquine plus atazanavir/ritonavir, Daru, doi:10.1007/s40199-020-00369-2
Rawson, Moore, Zhu, Ranganathan, Skolimowska et al., Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing, Clin. Infect. Dis, doi:10.1093/cid/ciaa530
Rees, Nightingale, Jafari, Waterlow, Clifford et al., COVID-19 length of hospital stay: A systematic review and data synthesis, BMC Med, doi:10.1186/s12916-020-01726-3
Rodriguez-Guerra, Jadhav, Vittorio, Current treatment in COVID-19 disease: A rapid review, Drugs Context, doi:10.7573/dic.2020-10-3
Rodríguez-Baño, Rossolini, Schultsz, Tacconelli, Murthy et al., Key considerations on the potential impacts of the COVID-19 pandemic on antimicrobial resistance research and surveillance, Trans. R. Soc. Trop. Med. Hyg, doi:10.1093/trstmh/trab048
Rosenberg, Dufort, Udo, Wilberschied, Kumar et al., Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State, JAMA, doi:10.1001/jama.2020.8630
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review, JAMA, doi:10.1001/jama.2020.6019
Schultze, Walker, Mackenna, Morton, Bhaskaran et al., Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: An observational cohort study using the OpenSAFELY platform, Lancet Respir. Med, doi:10.1016/S2213-2600(20)30415-X
Setiati, Azwar, COVID-19 and Indonesia, Acta Med. Indones
Shinkai, Tsushima, Tanaka, Hagiwara, Tarumoto et al., Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: A randomized, phase III clinical trial, Infect. Dis. Ther, doi:10.1007/s40121-021-00517-4
Silva, Estrela, Gomes, Piñeiro-Lamas, Figueiras et al., The impact of the COVID-19 pandemic on antibiotic prescribing trends in outpatient care: A nationwide, quasi-experimental approach, Antibiotics, doi:10.3390/antibiotics10091040
Sulis, Batomen, Kotwani, Pai, Gandra, Sales of antibiotics and hydroxychloroquine in India during the COVID-19 epidemic: An interrupted time series analysis, PLoS Med, doi:10.1371/journal.pmed.1003682
Surendra, Elyazar, Djaafara, Ekawati, Saraswati et al., Clinical characteristics and mortality associated with COVID-19 in Jakarta, Indonesia: A hospital-based retrospective cohort study, Lancet Reg. Health West Pac, doi:10.1016/j.lanwpc.2021.100108
Tavares, Mahadeshwar, Wan, Huston, Pyle, The global and local distribution of RNA structure throughout the SARS-CoV-2 genome, J. Virol, doi:10.1128/JVI.02190-20
Wang, Yang, Chen, Guo, Liu et al., The proportion and effect of corticosteroid therapy in patients with COVID-19 infection: A systematic review and meta-analysis, PLoS ONE, doi:10.1371/journal.pone.0249481
Wu, Li, Peng, Zhou, HIV protease inhibitors in gut barrier dysfunction and liver injury, Curr. Opin. Pharmacol, doi:10.1016/j.coph.2014.07.008
Wu, Wang, Kuo, Shannar, Peter et al., An update on current therapeutic drugs treating COVID-19, Curr. Pharmacol. Rep, doi:10.1007/s40495-020-00216-7
Yang, Liu, Zhou, Zhao, Zhao et al., The effect of corticosteroid treatment on patients with coronavirus infection: A systematic review and meta-analysis, J. Infect, doi:10.1016/j.jinf.2020.03.062
Yates, Razieh, Zaccardi, Rowlands, Seidu et al., Obesity, walking pace and risk of severe COVID-19 and mortality: Analysis of UK Biobank, Int. J. Obes, doi:10.1038/s41366-021-00771-z
Zanella, Zizioli, Castelli, Quiros-Roldan, Tenofovir, another inexpensive, well-known and widely available old drug repurposed for SARS-CoV-2 infection, Pharmaceuticals, doi:10.3390/ph14050454
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.3390/pathophysiology29010009",
"ISSN": [
"1873-149X"
],
"URL": "http://dx.doi.org/10.3390/pathophysiology29010009",
"abstract": "<jats:p>The clinical manifestations associated with COVID-19 disease is mainly due to a dysregulated host response related to the overexpression of inflammatory markers. Until recently, only remdesivir had gained FDA approval for COVID-19 hospitalized patients and there are currently no evidence-based therapeutic options or options for prevention of complications that have been established. Some medical treatments such as antivirals, antibacterials, antithrombotics, antipyretics, corticosteroids, interleukin inhibitors, monoclonal antibodies, convalescent plasma, immunostimulants, and vitamin supplements have been utilized. However, there are limited data to support their effectiveness. Hence, this study was attempted to identify and evaluate the effectiveness of antibacterials and antivirals used for COVID-19 using a retrospective cross-sectional approach based on the medical records of adult patients in four hospitals. The number of antibacterials was calculated in defined daily dose (DDD) per 100 bed-days unit. Both mixed-logit regression and analysis of covariance were used to determine the effectiveness of the aforementioned agents in relation to COVID-19 outcome and patients’ length of stay. The model was weighed accordingly and covariates (e.g., age) were considered in the model. Heart disease was found to be the most common pre-existing condition of COVID-19 hospitalized patients in this study. Azithromycin, an antibacterial in the Watch category list, was used extensively (33–65 DDD per 100 bed-days). Oseltamivir, an antiviral approved by the FDA for influenza was the most prescribed antiviral. In addition, favipiravir was found to be a significant factor in improving patients’ COVID-19 outcomes and decreasing their length of stay. This study strongly suggests that COVID-19 patients’ received polypharmacy for their treatment. However, most of the drugs used did not reach statistical significance in improving the patients’ condition or decreasing the length of stay. Further studies to support drug use are needed.</jats:p>",
"alternative-id": [
"pathophysiology29010009"
],
"author": [
{
"affiliation": [],
"family": "Yulia",
"given": "Rika",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ikasanti",
"given": "Putri Ayu Irma",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8355-955X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Herawati",
"given": "Fauna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hartono",
"given": "Ruddy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hanum",
"given": "Puri Safitri",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lestiono",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramdani",
"given": "Dewi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jaelani",
"given": "Abdul Kadir",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6417-8455",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kantono",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wijono",
"given": "Heru",
"sequence": "additional"
}
],
"container-title": "Pathophysiology",
"container-title-short": "Pathophysiology",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
3,
9
]
],
"date-time": "2022-03-09T06:53:54Z",
"timestamp": 1646808834000
},
"deposited": {
"date-parts": [
[
2022,
3,
9
]
],
"date-time": "2022-03-09T07:49:24Z",
"timestamp": 1646812164000
},
"funder": [
{
"DOI": "10.13039/501100012696",
"award": [
"Internal"
],
"doi-asserted-by": "publisher",
"name": "University of Surabaya"
}
],
"indexed": {
"date-parts": [
[
2022,
4,
1
]
],
"date-time": "2022-04-01T20:12:48Z",
"timestamp": 1648843968656
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
3,
7
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
7
]
],
"date-time": "2022-03-07T00:00:00Z",
"timestamp": 1646611200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1873-149X/29/1/9/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "92-105",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
3,
7
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
7
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"article-title": "COVID-19 and Indonesia",
"author": "Setiati",
"first-page": "84",
"journal-title": "Acta Med. Indones.",
"key": "ref1",
"volume": "52",
"year": "2020"
},
{
"DOI": "10.4269/ajtmh.20-1496",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1016/j.lanwpc.2021.100108",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.7573/dic.2020-10-3",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1001/jamanetworkopen.2021.16057",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.2147/IDR.S289037",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1177/2472555220958385",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1371/journal.pmed.1003501",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1001/jama.2020.8630",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1001/jama.2020.6019",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1007/s40495-020-00216-7",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1016/j.jinf.2020.03.062",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1371/journal.pone.0249481",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1016/j.jiph.2021.02.011",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1093/cid/ciaa530",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1371/journal.pone.0263437",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"key": "ref18",
"series-title": "Clinical Management of COVID-19 Interim Guidance—May 2020",
"year": "2020"
},
{
"DOI": "10.2471/BLT.20.268573",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.4102/ajlm.v9i1.1302",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1002/sim.5525",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"article-title": "Sample size calculation in medical studies",
"author": "Pourhoseingholi",
"first-page": "14",
"journal-title": "Gastroenterol. Hepatol. Bed. Bench.",
"key": "ref22",
"volume": "6",
"year": "2013"
},
{
"key": "ref23"
},
{
"DOI": "10.3390/antibiotics10020132",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"key": "ref25",
"unstructured": "ATC/DDD Index 2021https://www.whocc.no/atc_ddd_index/"
},
{
"key": "ref26",
"unstructured": "WHO International Working Group for Drug Statistics Methodology; WHO Collaborating Centre for Drug Statistics Methodology; WHO Collaborating Centre for Drug Utilization Research and Clinical Pharmacological Serviceshttp://www.who.int/iris/handle/10665/42627"
},
{
"key": "ref27",
"unstructured": "Tim COVID-19 IDAI. Pedoman Tatalaksana COVID-19 Edisi 2 Agustus 2020. Perhimpunan Dokter Paru Indonesia (PDPI), Perhimpunan Dokter Spesialis Kardiovaskular Indonesia (PERKI), Perhimpunan Dokter Spesialis Penyakit Dalam Indonesia (PAPDI), Perhimpunan Dokter Anestesiologi dan Terapi Intensif Indonesia (PERDATIN), Ikatan Dokter Anak Indonesia (IDAI). Tahun 2020https://www.papdi.or.id/pdfs/938/Pedoman%20Tatalaksana%20COVID-19%20edisi%202.pdf"
},
{
"key": "ref28",
"unstructured": "The 2019 WHO AWaRe Classification of Antibiotics for Evaluation and Monitoring of Usehttps://apps.who.int/iris/handle/10665/327957"
},
{
"key": "ref29",
"unstructured": "The AWaRe Classification of Antibiotics Databasehttps://adoptaware.org/"
},
{
"DOI": "10.1093/trstmh/trab048",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.1371/journal.pmed.1003682",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1016/j.phrs.2020.105403",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1016/j.jiph.2021.02.001",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.3390/antibiotics10091040",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1093/jacamr/dlab047",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1177/11786337211033870",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.1128/JVI.02190-20",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"key": "ref38"
},
{
"DOI": "10.3390/ph14050454",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.2174/1570162X15666171120110145",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1111/jcpt.13305",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1016/j.jiph.2021.07.019",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1056/NEJMoa2001282",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1136/bmjopen-2020-047016",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1016/j.coph.2014.07.008",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1007/s40264-020-00954-z",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1016/j.ijid.2020.10.069",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.1007/s40121-021-00517-4",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.1186/s12879-021-06164-x",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.1002/14651858.CD015025",
"doi-asserted-by": "publisher",
"key": "ref51"
},
{
"DOI": "10.3855/jidc.14404",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.1159/000512592",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"DOI": "10.18053/jctres.07.202103.010",
"doi-asserted-by": "publisher",
"key": "ref54"
},
{
"DOI": "10.2147/RMHP.S292538",
"doi-asserted-by": "publisher",
"key": "ref55"
},
{
"DOI": "10.5603/ARM.a2021.0036",
"doi-asserted-by": "publisher",
"key": "ref56"
},
{
"DOI": "10.2147/IJGM.S330580",
"doi-asserted-by": "publisher",
"key": "ref57"
},
{
"DOI": "10.1016/j.arcmed.2020.12.002",
"doi-asserted-by": "publisher",
"key": "ref58"
},
{
"DOI": "10.1371/journal.pone.0258154",
"doi-asserted-by": "publisher",
"key": "ref59"
},
{
"DOI": "10.3810/pgm.2009.11.2074",
"doi-asserted-by": "publisher",
"key": "ref60"
},
{
"DOI": "10.3389/fmed.2021.620044",
"doi-asserted-by": "publisher",
"key": "ref61"
},
{
"DOI": "10.1038/s41366-021-00771-z",
"doi-asserted-by": "publisher",
"key": "ref62"
},
{
"DOI": "10.1038/s41366-021-00872-9",
"doi-asserted-by": "publisher",
"key": "ref63"
},
{
"DOI": "10.1186/s12916-020-01726-3",
"doi-asserted-by": "publisher",
"key": "ref64"
},
{
"DOI": "10.1007/s40199-020-00369-2",
"doi-asserted-by": "publisher",
"key": "ref65"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"doi-asserted-by": "publisher",
"key": "ref66"
},
{
"DOI": "10.1186/s13223-020-00509-y",
"doi-asserted-by": "publisher",
"key": "ref67"
},
{
"key": "ref68",
"unstructured": "Risks of and from SARS-CoV-2 (COVID-19) Infection in People with Asthmahttps://www.cebm.net/covid-19/risks-of-and-from-sars-cov-2-covid-19-infection-in-people-with-asthma/"
},
{
"DOI": "10.1016/S2213-2600(20)30415-X",
"doi-asserted-by": "publisher",
"key": "ref69"
},
{
"DOI": "10.4103/lungindia.lungindia_965_20",
"doi-asserted-by": "publisher",
"key": "ref70"
}
],
"reference-count": 70,
"references-count": 70,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1873-149X/29/1/9"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Evaluation of Antibacterial and Antiviral Drug Effectiveness in COVID-19 Therapy: A Data-Driven Retrospective Approach",
"type": "journal-article",
"volume": "29"
}