Favipiravir in the Treatment of Outpatient COVID-19: A Multicenter, Randomized, Triple-Blind, Placebo-Controlled Clinical Trial
et al., Advances in Respiratory Medicine, doi:10.3390/arm91010004, IRCT20171219037964N3, Jan 2023
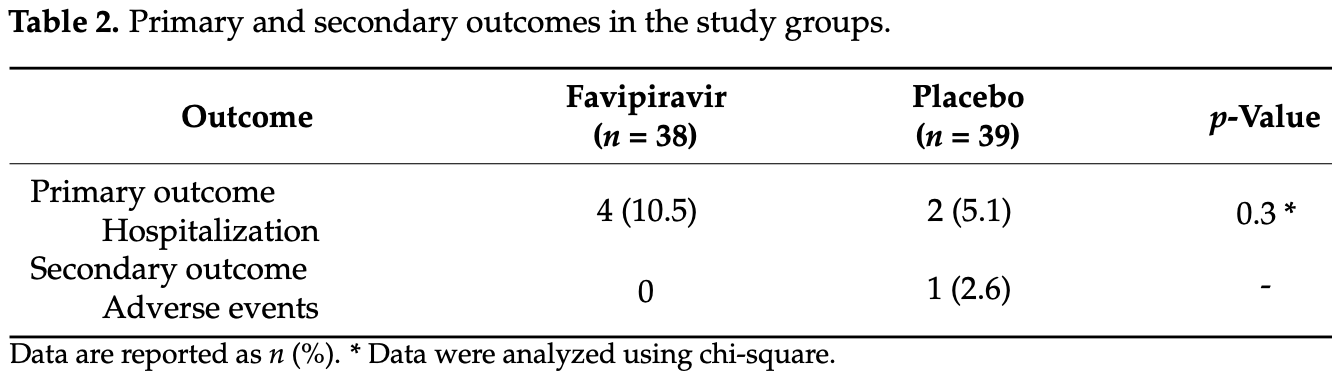
RCT 77 outpatients in Iran, showing increased hospitalization with treatment, without statistical significance. Favipiravir 1600mg daily for five days. 21% of favipiravir patients did not complete treatment.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
risk of hospitalization, 105.3% higher, RR 2.05, p = 0.43, treatment 4 of 38 (10.5%), control 2 of 39 (5.1%), day 28.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Vaezi et al., 28 Jan 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Iran, peer-reviewed, 6 authors, study period 5 December, 2020 - 31 March, 2021, trial IRCT20171219037964N3.
Contact: salahimehrdad@yahoo.com (corresponding author).
Favipiravir in the Treatment of Outpatient COVID-19: A Multicenter, Randomized, Triple-Blind, Placebo-Controlled Clinical Trial
Advances in Respiratory Medicine, doi:10.3390/arm91010004
What are the main findings?
• Favipiravir, an RNA-dependent RNA polymerase inhibitor, shows no benefit in preventing the hospitalization of mild to moderate COVID-19 patients. What is the implication of the main finding?
• Our results may inform decisions on the exclusion of Favipiravir from mild to moderate COVID-19 treatment guidelines.
Informed Consent Statement: Patients were informed about the study protocol and objectives and were asked to sign an informed consent before participation. All data were managed, analyzed, and reported anonymously. Medications were provided by the research team and imposed no costs on patients.
Conflicts of Interest: The authors declare that they have no conflict of interest.
References
Alqahtani, Kumar, Aljawder, Abdulrahman, Mohamed et al., Randomized controlled trial of favipiravir, hydroxychloroquine, and standard care in patients with mild/moderate COVID-19 disease, Sci. Rep, doi:10.1038/s41598-022-08794-w
Baranovich, Wong, Armstrong, Marjuki, Webby et al., T-705 (Favipiravir) Induces Lethal Mutagenesis in Influenza A H1N1 Viruses In Vitro, J. Virol, doi:10.1128/JVI.02346-12
Bosaeed, Alharbi, Mahmoud, Alrehily, Bahlaq et al., Efficacy of favipiravir in adults with mild COVID-19: A randomized, double-blind, multicentre, placebocontrolled clinical trial, Clin. Microbiol. Infect, doi:10.1016/j.cmi.2021.12.026
Bryant, Lawrie, Fordham, Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-analysis, and Trial Sequential Analysis to Inform Clinical Guidelines, Am. J. Ther, doi:10.1097/MJT.0000000000001402
Cai, Yang, Liu, Chen, Shu et al., Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study, Engineering, doi:10.1016/j.eng.2020.03.007
Darab, Keshavarz, Sadeghi, Shahmohamadi, Kavosi, The economic burden of coronavirus disease 2019 (COVID-19): Evidence from Iran, BMC Health Serv. Res
Deng, Yang, Yang, Chen, Qiu et al., Evaluation of favipiravir in the treatment of COVID-19 based on the real-world, Expert Rev. Anti-Infect. Ther, doi:10.1080/14787210.2022.2012155
Dessie, Zewotir, Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients, BMC Infect. Dis, doi:10.1186/s12879-021-06536-3
Frediansyah, Nainu, Dhama, Mudatsir, Harapan, Remdesivir and its antiviral activity against COVID-19: A systematic review, Clin. Epidemiology Glob. Health, doi:10.1016/j.cegh.2020.07.011
Furuta, Gowen, Takahashi, Shiraki, Smee et al., Favipiravir (T-705), a novel viral RNA polymerase inhibitor, Antivir. Res, doi:10.1016/j.antiviral.2013.09.015
Gonçalves, Bertrand, Ke, Comets, De Lamballerie et al., Timing of antiviral treatment initiation is critical to reduce SARS-CoV-2 viral load. CPT Pharmacomet, Syst. Pharmacol, doi:10.1002/psp4.12543
Goyal, Cardozo-Ojeda, Schiffer, Potency and timing of antiviral therapy as determinants of duration of SARS-CoV-2 shedding and intensity of inflammatory response, Sci. Adv, doi:10.1126/sciadv.abc7112
Hassaniazad, Farshidi, Gharibzadeh, Bazram, Khalili et al., Efficacy and safety of favipiravir plus interferon-beta versus lopinavir/ritonavir plus interferon-beta in moderately ill patients with COVID-19: A randomized clinical trial, J. Med. Virol, doi:10.1002/jmv.27724
Hassanipour, Arab-Zozani, Amani, Heidarzad, Fathalipour et al., Addendum: The efficacy and safety of Favipiravir in treatment of COVID-19: A systematic review and meta-analysis of clinical trials, Sci. Rep
Ivashchenko, Dmitriev, Vostokova, Azarova, Blinow et al., AVIFAVIR for Treatment of Patients With Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of a Phase II/III Multicenter Randomized Clinical Trial, Clin. Infect. Dis
Joshi, Parkar, Ansari, Vora, Talwar et al., Role of favipiravir in the treatment of COVID-19, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.10.069
Khamis, Al Naabi, Al Lawati, Ambusaidi, Al Sharji et al., Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.11.008
Kow, Ramachandram, Hasan, Future of antivirals in COVID-19: The case of favipiravir, Int. Immunopharmacol, doi:10.1016/j.intimp.2021.108455
Pan, Peto, Henao-Restrepo, Preziosi, Sathiyamoorthy et al., Repurposed Antiviral Drugs for COVID-19-Interim WHO Solidarity Trial Results, N. Engl. J. Med
Reddy, Patil, Khobragade, Balki, Raj et al., Evaluation of the Safety and Efficacy of Favipiravir in Adult Indian Patients with Mild-to-Moderate COVID-19 in a Real-World Setting, Int. J. Gen. Med, doi:10.2147/IJGM.S349241
Schulz, Altman, Moher, Consort, statement: Updated guidelines for reporting parallel group randomised trials, J. Pharmacol. Pharmacother, doi:10.4103/0976-500X.72352
Shrestha, Budhathoki, Khadka, Shah, Pokharel et al., Favipiravir versus other antiviral or standard of care for COVID-19 treatment: A rapid systematic review and meta-analysis, Virol. J, doi:10.1186/s12985-020-01412-z
Sirijatuphat, Manosuthi, Niyomnaitham, Owen, Copeland et al., Early treatment of Favipiravir in COVID-19 patients without pneumonia: A multicentre, open-labelled, randomized control study, Emerg. Microbes Infect, doi:10.1080/22221751.2022.2117092
Solaymani-Dodaran, Ghanei, Bagheri, Qazvini, Vahedi et al., Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia, Int. Immunopharmacol, doi:10.1016/j.intimp.2021.107522
Soy, Keser, Atagündüz, Tabak, Atagündüz et al., Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment, Clin. Rheumatol, doi:10.1007/s10067-020-05190-5
Worldometer, COVID-19 Pandemic
DOI record:
{
"DOI": "10.3390/arm91010004",
"ISSN": [
"2543-6031"
],
"URL": "http://dx.doi.org/10.3390/arm91010004",
"abstract": "<jats:p>Background: Finding effective outpatient treatments to prevent COVID-19 progression and hospitalization is necessary and is helpful in managing limited hospital resources. Repurposing previously existing treatments is highly desirable. In this study, we evaluate the efficacy of Favipiravir in the prevention of hospitalization in symptomatic COVID-19 patients who were not eligible for hospitalization. Methods: This study was a triple-blind randomized controlled trial conducted between 5 December 2020 and 31 March 2021 in three outpatient centers in Isfahan, Iran. Patients in the intervention group received Favipiravir 1600 mg daily for five days, and the control group received a placebo. Our primary outcome was the proportion of hospitalized participants from day 0 to day 28. The outcome was assessed on days 3, 7, 14, 21, and 28 through phone calls. Results: Seventy-seven patients were randomly allocated to Favipiravir and placebo groups. There was no significant difference between groups considering baseline characteristics. During the study period, 10.5% of patients in the Favipiravir group and 5.1% of patients in the placebo group were hospitalized, but there was no significant difference between them (p-value = 0.3). No adverse event was reported in the treatment group. Conclusions: Our study shows that Favipiravir did not reduce the hospitalization rate of mild to moderate COVID-19 patients in outpatient settings.</jats:p>",
"alternative-id": [
"arm91010004"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-1829-0494",
"affiliation": [
{
"name": "Cancer Prevention Research Center, Isfahan University of Medical Sciences, Isfahan 8174673461, Iran"
}
],
"authenticated-orcid": false,
"family": "Vaezi",
"given": "Atefeh",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, School of Medicine, Isfahan University of Medical Sciences, Isfahan 8174673461, Iran"
}
],
"family": "Salmasi",
"given": "Mehrzad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Bamdad Respiratory and Sleep Research Center, Department of Internal Medicine, School of Medicine, Isfahan University of Medical Sciences, Isfahan 8174673461, Iran"
}
],
"family": "Soltaninejad",
"given": "Forogh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Disease, School of Medicine, Isfahan University of Medical Sciences, Isfahan 8174673461, Iran"
}
],
"family": "Salahi",
"given": "Mehrdad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Physiology, Applied Physiology Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan 8174673461, Iran"
}
],
"family": "Javanmard",
"given": "Shaghayegh Haghjooy",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9606-7434",
"affiliation": [
{
"name": "Bamdad Respiratory and Sleep Research Center, Department of Internal Medicine, School of Medicine, Isfahan University of Medical Sciences, Isfahan 8174673461, Iran"
}
],
"authenticated-orcid": false,
"family": "Amra",
"given": "Babak",
"sequence": "additional"
}
],
"container-title": "Advances in Respiratory Medicine",
"container-title-short": "Advances in Respiratory Medicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
1,
30
]
],
"date-time": "2023-01-30T16:25:47Z",
"timestamp": 1675095947000
},
"deposited": {
"date-parts": [
[
2023,
1,
30
]
],
"date-time": "2023-01-30T18:19:29Z",
"timestamp": 1675102769000
},
"funder": [
{
"name": "Isfahan University of Medical Sciences"
}
],
"indexed": {
"date-parts": [
[
2023,
1,
31
]
],
"date-time": "2023-01-31T06:18:41Z",
"timestamp": 1675145921178
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
1,
28
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
28
]
],
"date-time": "2023-01-28T00:00:00Z",
"timestamp": 1674864000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2543-6031/91/1/4/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "18-25",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
1,
28
]
]
},
"published-online": {
"date-parts": [
[
2023,
1,
28
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref_1",
"unstructured": "Worldometer (2022, October 01). COVID-19 Pandemic. Available online: https://www.worldometers.info/coronavirus/."
},
{
"DOI": "10.1186/s12879-021-06536-3",
"doi-asserted-by": "crossref",
"key": "ref_2",
"unstructured": "Dessie, Z.G., and Zewotir, T. (2021). Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis., 21."
},
{
"article-title": "The economic burden of coronavirus disease 2019 (COVID-19): Evidence from Iran",
"author": "Darab",
"first-page": "1",
"journal-title": "BMC Health Serv. Res.",
"key": "ref_3",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1126/sciadv.abc7112",
"article-title": "Potency and timing of antiviral therapy as determinants of duration of SARS-CoV-2 shedding and intensity of inflammatory response",
"author": "Goyal",
"doi-asserted-by": "crossref",
"first-page": "eabc7112",
"journal-title": "Sci. Adv.",
"key": "ref_4",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1002/psp4.12543",
"article-title": "Timing of antiviral treatment initiation is critical to reduce SARS-CoV-2 viral load",
"author": "Bertrand",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "CPT Pharmacomet. Syst. Pharmacol.",
"key": "ref_5",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1007/s10067-020-05190-5",
"article-title": "Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment",
"author": "Soy",
"doi-asserted-by": "crossref",
"first-page": "2085",
"journal-title": "Clin. Rheumatol.",
"key": "ref_6",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1097/MJT.0000000000001402",
"article-title": "Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-analysis, and Trial Sequential Analysis to Inform Clinical Guidelines",
"author": "Bryant",
"doi-asserted-by": "crossref",
"first-page": "e434",
"journal-title": "Am. J. Ther.",
"key": "ref_7",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1016/j.cegh.2020.07.011",
"article-title": "Remdesivir and its antiviral activity against COVID-19: A systematic review",
"author": "Frediansyah",
"doi-asserted-by": "crossref",
"first-page": "123",
"journal-title": "Clin. Epidemiology Glob. Health",
"key": "ref_8",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2023184",
"article-title": "Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results",
"author": "Pan",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "N. Engl. J. Med.",
"key": "ref_9",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1038/s41598-022-05835-2",
"article-title": "Addendum: The efficacy and safety of Favipiravir in treatment of COVID-19: A systematic review and meta-analysis of clinical trials",
"author": "Hassanipour",
"doi-asserted-by": "crossref",
"first-page": "1996",
"journal-title": "Sci. Rep.",
"key": "ref_10",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1128/JVI.02346-12",
"article-title": "T-705 (Favipiravir) Induces Lethal Mutagenesis in Influenza A H1N1 Viruses In Vitro",
"author": "Baranovich",
"doi-asserted-by": "crossref",
"first-page": "3741",
"journal-title": "J. Virol.",
"key": "ref_11",
"volume": "87",
"year": "2013"
},
{
"DOI": "10.1016/j.antiviral.2013.09.015",
"article-title": "Favipiravir (T-705), a novel viral RNA polymerase inhibitor",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "446",
"journal-title": "Antivir. Res.",
"key": "ref_12",
"volume": "100",
"year": "2013"
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"article-title": "Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study",
"author": "Cai",
"doi-asserted-by": "crossref",
"first-page": "1192",
"journal-title": "Engineering",
"key": "ref_13",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/j.intimp.2021.107522",
"article-title": "Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia",
"author": "Ghanei",
"doi-asserted-by": "crossref",
"first-page": "107522",
"journal-title": "Int. Immunopharmacol.",
"key": "ref_14",
"volume": "95",
"year": "2021"
},
{
"DOI": "10.4103/0976-500X.72352",
"article-title": "CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials",
"author": "Schulz",
"doi-asserted-by": "crossref",
"first-page": "100",
"journal-title": "J. Pharmacol. Pharmacother.",
"key": "ref_15",
"volume": "1",
"year": "2010"
},
{
"DOI": "10.1002/jmv.27724",
"article-title": "Efficacy and safety of favipiravir plus interferon-beta versus lopinavir/ritonavir plus interferon-beta in moderately ill patients with COVID-19: A randomized clinical trial",
"author": "Hassaniazad",
"doi-asserted-by": "crossref",
"first-page": "3184",
"journal-title": "J. Med. Virol.",
"key": "ref_16",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2020.11.008",
"article-title": "Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia",
"author": "Khamis",
"doi-asserted-by": "crossref",
"first-page": "538",
"journal-title": "Int. J. Infect. Dis.",
"key": "ref_17",
"volume": "102",
"year": "2021"
},
{
"DOI": "10.1038/s41598-022-08794-w",
"article-title": "Randomized controlled trial of favipiravir, hydroxychloroquine, and standard care in patients with mild/moderate COVID-19 disease",
"author": "AlQahtani",
"doi-asserted-by": "crossref",
"first-page": "4925",
"journal-title": "Sci. Rep.",
"key": "ref_18",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciaa1176",
"article-title": "AVIFAVIR for Treatment of Patients With Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of a Phase II/III Multicenter Randomized Clinical Trial",
"author": "Ivashchenko",
"doi-asserted-by": "crossref",
"first-page": "531",
"journal-title": "Clin. Infect. Dis.",
"key": "ref_19",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1080/22221751.2022.2117092",
"article-title": "Early treatment of Favipiravir in COVID-19 patients without pneumonia: A multicentre, open-labelled, randomized control study",
"author": "Sirijatuphat",
"doi-asserted-by": "crossref",
"first-page": "2197",
"journal-title": "Emerg. Microbes Infect.",
"key": "ref_20",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2021.12.026",
"article-title": "Efficacy of favipiravir in adults with mild COVID-19: A randomized, double-blind, multicentre, placebo-controlled clinical trial",
"author": "Bosaeed",
"doi-asserted-by": "crossref",
"first-page": "602",
"journal-title": "Clin. Microbiol. Infect.",
"key": "ref_21",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1080/14787210.2022.2012155",
"article-title": "Evaluation of favipiravir in the treatment of COVID-19 based on the real-world",
"author": "Deng",
"doi-asserted-by": "crossref",
"first-page": "555",
"journal-title": "Expert Rev. Anti-Infect. Ther.",
"key": "ref_22",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1186/s12985-020-01412-z",
"article-title": "Favipiravir versus other antiviral or standard of care for COVID-19 treatment: A rapid systematic review and meta-analysis",
"author": "Shrestha",
"doi-asserted-by": "crossref",
"first-page": "141",
"journal-title": "Virol. J.",
"key": "ref_23",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1016/j.intimp.2021.108455",
"article-title": "Future of antivirals in COVID-19: The case of favipiravir",
"author": "Kow",
"doi-asserted-by": "crossref",
"first-page": "108455",
"journal-title": "Int. Immunopharmacol.",
"key": "ref_24",
"volume": "103",
"year": "2022"
},
{
"DOI": "10.2147/IJGM.S349241",
"article-title": "Evaluation of the Safety and Efficacy of Favipiravir in Adult Indian Patients with Mild-to-Moderate COVID-19 in a Real-World Setting",
"author": "Reddy",
"doi-asserted-by": "crossref",
"first-page": "4551",
"journal-title": "Int. J. Gen. Med.",
"key": "ref_25",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2020.10.069",
"article-title": "Role of favipiravir in the treatment of COVID-19",
"author": "Joshi",
"doi-asserted-by": "crossref",
"first-page": "501",
"journal-title": "Int. J. Infect. Dis.",
"key": "ref_26",
"volume": "102",
"year": "2021"
}
],
"reference-count": 26,
"references-count": 26,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2543-6031/91/1/4"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": "Favipiravir in the Treatment of Outpatient COVID-19: A Multicenter, Randomized, Triple-Blind, Placebo-Controlled Clinical Trial",
"type": "journal-article",
"volume": "91"
}