Efficacy and safety of favipiravir plus interferon-beta versus lopinavir/ritonavir plus interferon-beta in moderately ill patients with COVID-19: A randomized clinical trial
et al., Journal of Medical Virology, doi:10.1002/jmv.27724, IRCT20200506047323N3, Mar 2022
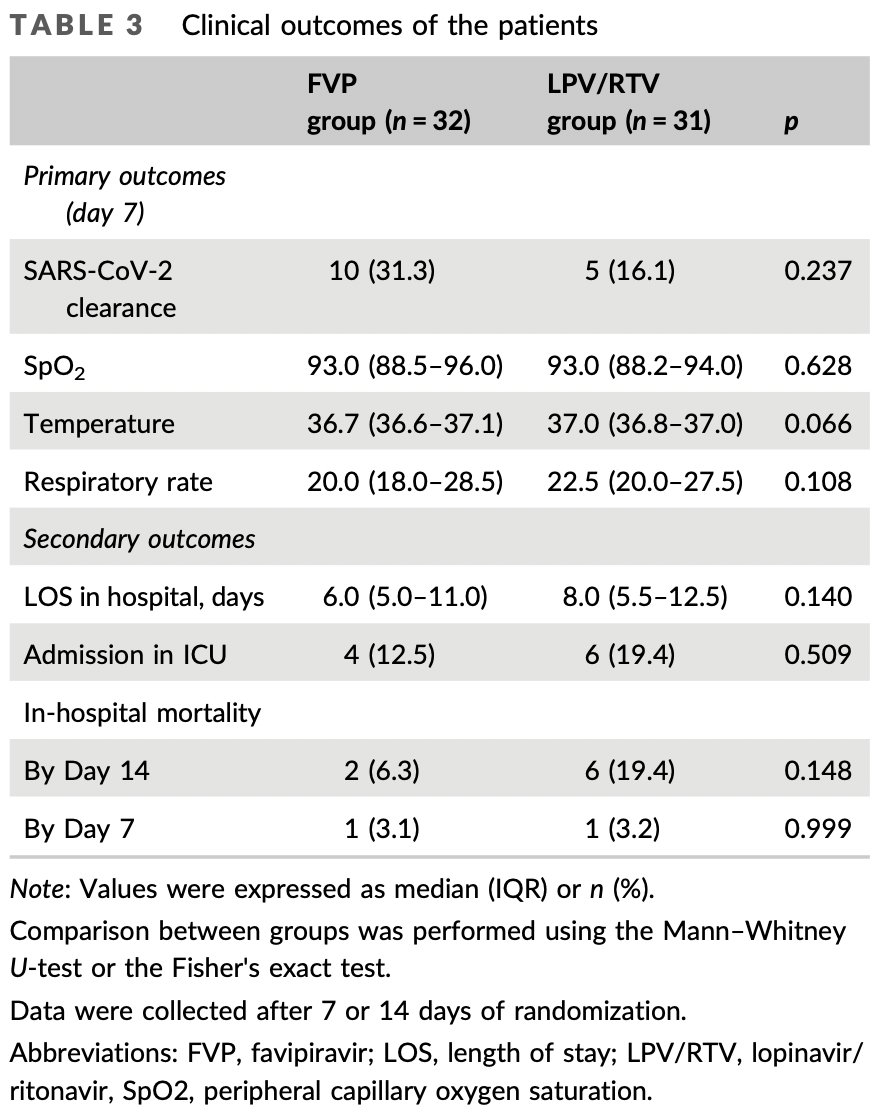
RCT comparing favipiravir and lopinavir/ritonavir, showing no significant differences. All patients received interferon-beta. Favipiravir 1600mg bid for the first day and 600mg bid for the following 4 days.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
risk of death, 67.7% lower, RR 0.32, p = 0.15, treatment 2 of 32 (6.2%), control 6 of 31 (19.4%), NNT 7.6, day 14.
|
|
risk of death, 3.1% lower, RR 0.97, p = 1.00, treatment 1 of 32 (3.1%), control 1 of 31 (3.2%), NNT 992, day 7.
|
|
risk of ICU admission, 35.4% lower, RR 0.65, p = 0.51, treatment 4 of 32 (12.5%), control 6 of 31 (19.4%), NNT 15, day 14.
|
|
hospitalization time, 25.0% lower, relative time 0.75, p = 0.14, treatment 32, control 31.
|
|
risk of no viral clearance, 18.0% lower, RR 0.82, p = 0.24, treatment 22 of 32 (68.8%), control 26 of 31 (83.9%), NNT 6.6, day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Hassaniazad et al., 24 Mar 2022, Randomized Controlled Trial, Iran, peer-reviewed, mean age 53.8, 7 authors, this trial compares with another treatment - results may be better when compared to placebo, trial IRCT20200506047323N3.
Contact: m.fathalipour@yahoo.com.
Efficacy and safety of favipiravir plus interferon‐beta versus lopinavir/ritonavir plus interferon‐beta in moderately ill patients with COVID‐19: A randomized clinical trial
Journal of Medical Virology, doi:10.1002/jmv.27724
Favipiravir (FVP), lopinavir/ritonavir (LPV/RTV), and interferon-beta (INF-beta) are considered as potential treatments for COVID-19. We examined the efficacy and safety of FVP and INF-beta compared to LPV/RTV and INF-beta combinations for the treatment of SARS-CoV-2. It was a single-center randomized clinical trial. Eligible patients were randomized to receive FVP plus INF-beta versus LPV/RTV plus INF-beta. The primary endpoint was the viral clearance after seven days of randomization. ICU admission, length of stay (LOS) in hospital, in-hospital mortality, and the incidence of adverse events were also measured. This trial was registered on the Iranian Registry of Clinical Trials (IRCT20200506047323N3). Patients were randomly allocated to the FVP (n = 33) and LPV/RTV (n = 33) groups. The viral clearance on Day seven was not significantly different between the FVP (31.1%) and the LPV/RTV groups (16.1%). The rate of ICU admission and likewise the in-hospital mortality in the FVP group (12.5% and 6.3%, respectively) were similar to the LPV/RTV groups (19.4% and 19.4%, respectively). The median LOS in the hospital was also not different (6.8 days [interquartile range; IQR = 5.0-11.0] in the FVP and (8.0 days [IQR = 5.5-12.5]) in LPV/RTV groups (p = 0.140). Adverse events were observed in 25.0% of FVP and 32.3% of LPV/RTV groups. The combination therapy with FVP did not exert a higher efficacy compared to the combination regimen of LPV/RTV. However, both treatment regimens demonstrated a mild profile of adverse events.
ACKNOWLEDGMENTS The authors appreciably thank the trial patients and their families, whose help and participation made this study possible. This study was financially supported by Hormozgan University of Medical Sciences (Grant no: 990233).
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
SUPPORTING INFORMATION Additional supporting information may be found in the online version of the article at the publisher's website.
References
Alhumaid, Mutair, Alawi, Alhmeed, Zaidi et al., Efficacy and safety of lopinavir/ritonavir for treatment of covid-19: a systematic review and meta-analysis, Trop Med Infect Dis
Cai, Yang, Liu, Experimental treatment with favipiravir for COVID-19: an open-label control study, Engineering
Chen, Zhang, Huang, Favipiravir versus Arbidol for COVID-19: a Randomized Clinical Trial, medRxiv, doi:10.1101/2020.03.17.20037432
Dabbous, El-Sayed, Assal, A randomized controlled study of favipiravir vs hydroxychloroquine in COVID-19 management: what have we learned so far?, Res Sq
Davoudi-Monfared, Rahmani, Khalili, A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19, Antimicrob Agents Chemother
Doi, Hibino, Hase, A prospective, randomized, openlabel trial of early versus late favipiravir therapy in hospitalized patients with COVID-19, Antimicrob Agents Chemother
Erdem, Ekren, Çağlayan, Treatment of SARS-CoV-2 pneumonia with favipiravir: early results from the Ege University cohort, Turkey, Turk J Med Sci
Garnier, Vaucher, Bianchi, Organizational impacts and clinical challenges of the COVID-19 pandemic on a Swiss Tertiary Internal Medicine Department, Rev Med Suisse
Hassaniazad, Bazram, Hassanipour, Fathalipour, Evaluation of the efficacy and safety of favipiravir and interferon compared to lopinavir/ritonavir and interferon in moderately ill patients with COVID-19: a structured summary of a study protocol for a randomized controlled trial, Trials
Hassanipour, Arab-Zozani, Amani, Heidarzad, Fathalipour et al., The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials, Sci Rep
Joseph, Dibas, Evanson, Efficacy and safety of lopinavir/ritonavir in the treatment of COVID-19: a systematic review, Expert Rev Anti Infect Ther
Khamis, Naabi, Lawati, Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia, Int J Infect Dis
Lou, Liu, Yao, Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID-19 patients: an exploratory randomized, controlled trial, Eur J Pharm Sci
Pilkington, Pepperrell, Hill, A review of the safety of favipiravir -a potential treatment in the COVID-19 pandemic?, J Virus Erad
Romera-Liebana, Orfila, Segura, Effects of a primary care-based multifactorial intervention on physical and cognitive function in frail, elderly individuals: a randomized controlled trial, J Gerontol A Biol Sci Med Sci
Shrestha, Budhathoki, Khadka, Shah, Pokharel et al., Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis, Virol J
Udwadia, Singh, Barkate, Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int J Infect Dis
Zhao, Zhu, Zhang, Tocilizumab combined with favipiravir in the treatment of COVID-19: a multicenter trial in a small sample size, Biomed Pharmacother
DOI record:
{
"DOI": "10.1002/jmv.27724",
"ISSN": [
"0146-6615",
"1096-9071"
],
"URL": "http://dx.doi.org/10.1002/jmv.27724",
"alternative-id": [
"10.1002/jmv.27724"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-11-01"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-03-14"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-03-24"
}
],
"author": [
{
"affiliation": [
{
"name": "Infectious and Tropical Diseases Research Center, Hormozgan Health Institute Hormozgan University of Medical Sciences Bandar Abbas Iran"
}
],
"family": "Hassaniazad",
"given": "Mehdi",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Cardiovascular Research Center Hormozgan University of Medical Sciences Bandar Abbas Iran"
}
],
"family": "Farshidi",
"given": "Hossein",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Social Determinants in Health Promotion Research Center, Hormozgan Health Institute Hormozgan University of Medical Sciences Bandar Abbas Iran"
}
],
"family": "Gharibzadeh",
"given": "Abdollah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious and Tropical Diseases Research Center, Hormozgan Health Institute Hormozgan University of Medical Sciences Bandar Abbas Iran"
}
],
"family": "Bazram",
"given": "Ali",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8354-9426",
"affiliation": [
{
"name": "Student Research Committee Hormozgan University of Medical Sciences Bandar Abbas Iran"
}
],
"authenticated-orcid": false,
"family": "Khalili",
"given": "Elham",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Inpatient Services Iran Food and Drug Administration Tehran Iran"
}
],
"family": "Noormandi",
"given": "Afsaneh",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4568-7024",
"affiliation": [
{
"name": "Department of Pharmacology and Toxicology, Faculty of Pharmacy Hormozgan University of Medical Sciences Bandar Abbas Iran"
},
{
"name": "Endocrinology and Metabolic Research Center Hormozgan University of Medical Sciences Bandar Abbas Iran"
}
],
"authenticated-orcid": false,
"family": "Fathalipour",
"given": "Mohammad",
"sequence": "additional"
}
],
"container-title": "Journal of Medical Virology",
"container-title-short": "Journal of Medical Virology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
3,
16
]
],
"date-time": "2022-03-16T08:26:15Z",
"timestamp": 1647419175000
},
"deposited": {
"date-parts": [
[
2022,
3,
24
]
],
"date-time": "2022-03-24T21:29:29Z",
"timestamp": 1648157369000
},
"funder": [
{
"DOI": "10.13039/501100011917",
"doi-asserted-by": "publisher",
"name": "Hormozgan University of Medical Sciences"
}
],
"indexed": {
"date-parts": [
[
2022,
4,
2
]
],
"date-time": "2022-04-02T21:06:05Z",
"timestamp": 1648933565306
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
3,
24
]
]
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
24
]
],
"date-time": "2022-03-24T00:00:00Z",
"timestamp": 1648080000000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
24
]
],
"date-time": "2022-03-24T00:00:00Z",
"timestamp": 1648080000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.27724",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/jmv.27724",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.27724",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2022,
3,
24
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
24
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/j.eng.2020.03.007",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"article-title": "A prospective, randomized, open‐label trial of early versus late favipiravir therapy in hospitalized patients with COVID‐19",
"author": "Doi Y",
"first-page": "12",
"journal-title": "Antimicrob Agents Chemother",
"key": "e_1_2_10_3_1",
"volume": "64",
"year": "2020"
},
{
"article-title": "Favipiravir versus Arbidol for COVID‐19: a Randomized Clinical Trial",
"author": "Chen C",
"journal-title": "medRxiv",
"key": "e_1_2_10_4_1",
"year": "2020"
},
{
"DOI": "10.1016/j.biopha.2020.110825",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_5_1"
},
{
"DOI": "10.1093/gerona/glx259",
"article-title": "Effects of a primary care‐based multifactorial intervention on physical and cognitive function in frail, elderly individuals: a randomized controlled trial",
"author": "Romera‐Liebana L",
"doi-asserted-by": "crossref",
"first-page": "1688",
"issue": "12",
"journal-title": "J Gerontol A Biol Sci Med Sci",
"key": "e_1_2_10_6_1",
"volume": "73",
"year": "2018"
},
{
"DOI": "10.1080/14787210.2021.1848545",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"article-title": "Efficacy and safety of lopinavir/ritonavir for treatment of covid‐19: a systematic review and meta‐analysis",
"author": "Alhumaid S",
"first-page": "4",
"journal-title": "Trop Med Infect Dis",
"key": "e_1_2_10_8_1",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.11.008",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.1128/AAC.01061-20",
"article-title": "A randomized clinical trial of the efficacy and safety of interferon β‐1a in treatment of severe COVID‐19",
"author": "Davoudi‐Monfared E",
"doi-asserted-by": "crossref",
"first-page": "9",
"journal-title": "Antimicrob Agents Chemother",
"key": "e_1_2_10_10_1",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1016/j.ejps.2020.105631",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_1"
},
{
"DOI": "10.1186/s13063-020-04747-8",
"article-title": "Evaluation of the efficacy and safety of favipiravir and interferon compared to lopinavir/ritonavir and interferon in moderately ill patients with COVID‐19: a structured summary of a study protocol for a randomized controlled trial",
"author": "Hassaniazad M",
"doi-asserted-by": "crossref",
"first-page": "886",
"issue": "1",
"journal-title": "Trials",
"key": "e_1_2_10_12_1",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.3906/sag-2008-33",
"article-title": "Treatment of SARS‐CoV‐2 pneumonia with favipiravir: early results from the Ege University cohort, Turkey",
"author": "Erdem HA",
"doi-asserted-by": "crossref",
"first-page": "912",
"issue": "3",
"journal-title": "Turk J Med Sci",
"key": "e_1_2_10_13_1",
"volume": "51",
"year": "2021"
},
{
"DOI": "10.1186/s12985-020-01412-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_14_1"
},
{
"article-title": "Organizational impacts and clinical challenges of the COVID‐19 pandemic on a Swiss Tertiary Internal Medicine Department",
"author": "Garnier A",
"first-page": "869",
"issue": "691",
"journal-title": "Rev Med Suisse",
"key": "e_1_2_10_15_1",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.1038/s41598-021-90551-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_1"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"article-title": "A randomized controlled study of favipiravir vs hydroxychloroquine in COVID‐19 management: what have we learned so far?",
"author": "Dabbous HM",
"journal-title": "Res Sq",
"key": "e_1_2_10_18_1",
"year": "2020"
},
{
"DOI": "10.1016/S2055-6640(20)30016-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/jmv.27724"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": "Efficacy and safety of favipiravir plus interferon‐beta versus lopinavir/ritonavir plus interferon‐beta in moderately ill patients with COVID‐19: A randomized clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}