Clinical effectiveness, safety, and viral mutagenicity of oral favipiravir for COVID-19: results from a community-based, open-label, randomized Phase III trial
et al., Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.00054-25, GETAFIX, ISRCTN31062548, Jun 2025
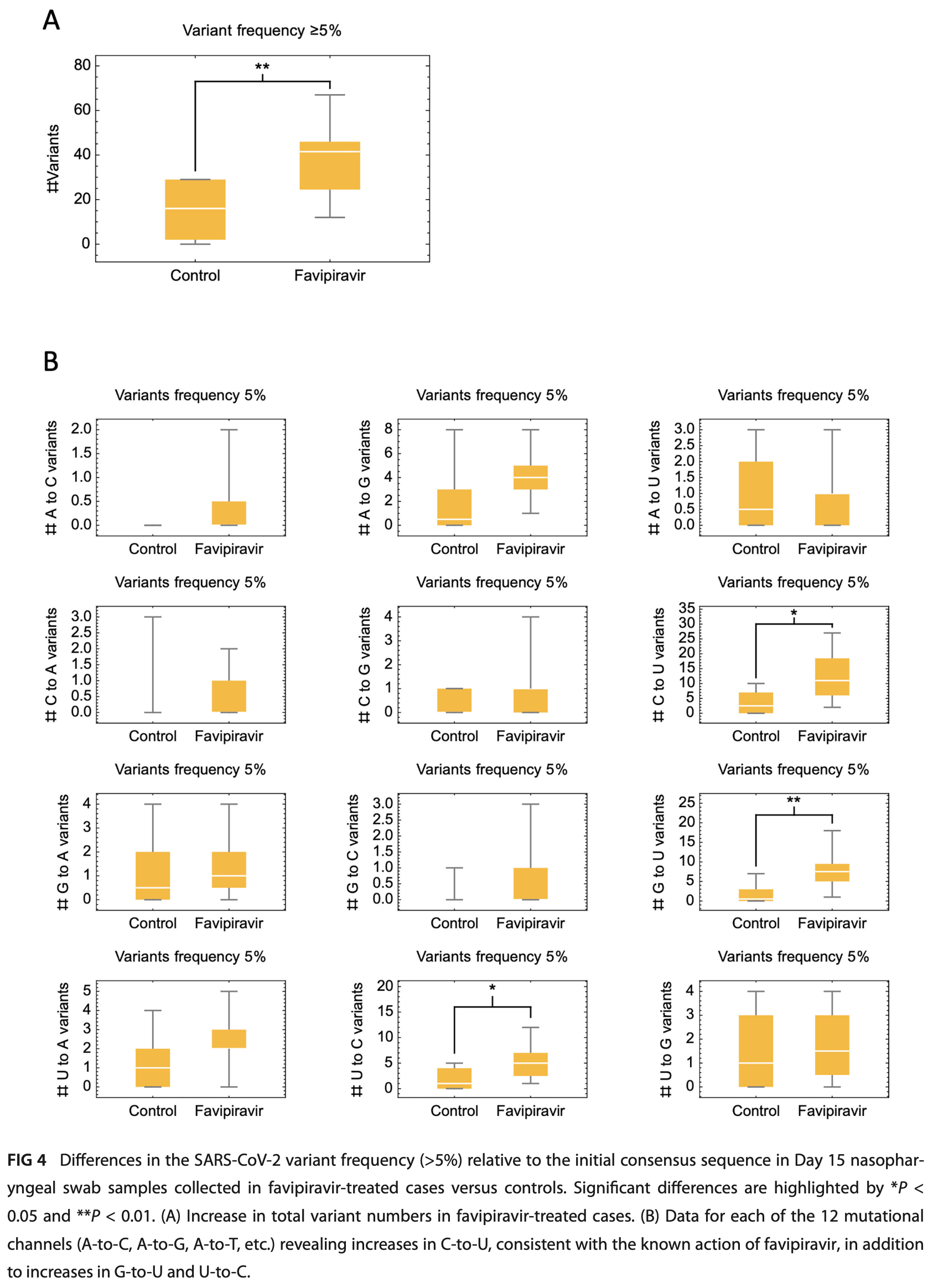
RCT 302 outpatients with mild COVID-19 showing no significant difference in outcomes with favipiravir treatment. The study population was relatively young and had few comorbidities, resulting in a low incidence of severe disease. Favipiravir was associated with increased SARS-CoV-2 viral mutagenicity, particularly C-to-U mutations.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments15.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of hospitalization, 34.2% lower, RR 0.66, p = 0.68, treatment 2 of 152 (1.3%), control 3 of 150 (2.0%), NNT 146.
|
|
risk of 7-point scale, 20.6% lower, OR 0.79, p = 0.61, treatment 152, control 150, adjusted per study, inverted to make OR<1 favor treatment, clinical status to day 15, RR approximated with OR.
|
|
risk of no recovery, 2.9% lower, HR 0.97, p = 0.82, treatment 152, control 150, inverted to make HR<1 favor treatment, time to symptom resolution.
|
|
risk of no viral clearance, 11.5% lower, HR 0.88, p = 0.68, treatment 152, control 150, inverted to make HR<1 favor treatment, time to viral clearance.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
13.
Cenikli et al., Does Favipiravir interact with DNA? Design of electrochemical DNA nanobiosensor to investigate the interaction between DNA and Favipiravir used in the treatment of COVID-19, Talanta, doi:10.1016/j.talanta.2025.128084.
Tate et al., 24 Jun 2025, Randomized Controlled Trial, United Kingdom, peer-reviewed, mean age 47.2, 39 authors, study period December 2020 - July 2022, average treatment delay 3.84 days, trial ISRCTN31062548 (GETAFIX).
Contact: kevin.blyth@glasgow.ac.uk.
Clinical effectiveness, safety, and viral mutagenicity of oral favipiravir for COVID-19: results from a community-based, open-label, randomized Phase III trial
Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.00054-25
Early community treatment of severe acute respiratory syndrome coro navirus-2 (SARS-CoV-2) infection may reduce severe coronavirus disease (COVID-19) incidence. We evaluated clinical effectiveness, safety, and SARS-CoV-2 mutagenicity of favipiravir, an oral viral RNA polymerase inhibitor. We performed an open-label, community-based, randomized Phase III trial, recruiting non-hospitalized adults with mild COVID-19 (WHO ordinal severity score [OSS] ≤ 3). Positive cases were invited to web-based self-screening within 24 h using public health data. Exclusion criteria included symptoms for >7 days, pregnancy/breastfeeding, severe renal/liver disease, gout, and licensed antiviral eligibility. Participants were randomized 1:1 to 10 days favipiravir (Day 1: 3,600 mg; days 2-10: 1,600 mg) or no additional treatment. The primary endpoint was worst recorded OSS up to and including Day 15 (intention-totreat). The target recruitment was 302. Secondary endpoints included adverse event (AE) rate to Day 60, time-to-viral clearance (TTVC), time-to-symptom resolution (TTSR), and SARS-CoV-2 sequencing variant rate (≥5% frequency) at Day 15 (registration ISRCTN: 31062548; EudraCT: 2020-001904-41). A total of 68,788 adults were invited, and 302 (0.4%) were subsequently randomized between December 2020 and July 2022 (favipiravir [n = 152]: standard care [n = 150]). Mean (SD) age was 47.2 (13.2), and 230/302 (76%) were vaccinated. Severe outcomes were infrequent, with no intensive care unit admissions/deaths. There was no difference in the primary endpoint: odds ratio 1.18 (95% confidence interval [CI] 0.63-2.20), TTSR (HR 1.03 [95% CI 0.81-1.31]), or TTVC (HR 1.13 [95% CI 0.65-1.97]). Favipiravir was well tolerated with few AEs but was associated with increased variant frequency, including C-to-U mutations. Community administration of favipiravir for mild COVID-19 was not associated with clinical benefits or safety concerns but was associated with SARS-CoV-2 mutagenicity. CLINICAL TRIALS This study is registered with ISRCTN as 31062548 and with EU-CTR as 2020-001904-41. KEYWORDS COVID-19, antiviral agents, randomized controlled trial S evere acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection remains as a significant public health concern (1). Widespread vaccination has reduced the incidence of severe coronavirus disease (COVID-19) (2), but infection continues to cause substantial morbidity, particularly in patients with impaired immunity and in communities with poor vaccine uptake (3). This places a premium on the develop ment of additional strategies to minimize the impact of future infection waves (4).
AUTHOR AFFILIATIONS
ETHICS APPROVAL The trial protocol was approved by the West of Scotland Research Ethics Com mittee (20/WS/0073) and UK Medicines & Healthcare Products Regulatory Agency (CTA24712/0052/001-0001). NHSGGC and the University of Glasgow co-sponsored the trial. The NHSGGC Caldicott Guardian approved sharing of test data by PHS. Recruitment, primary endpoint, and safety data were reviewed by an Independent Data Monitoring & Safety Committee chaired by C.B.
ADDITIONAL FILES The following material is available online.
Supplemental Material Supplementary material (AAC00054-25-S0001.docx). Fig. S1 to S4; Tables S1 to S5 .
References
Bray, Sopwith, Edmunds, Vansteenhouse, Feenstra et al., RT-PCR genotyping assays to identify SARS-CoV-2 variants in England in 2021: a design and retrospective evaluation study, Lancet Microbe, doi:10.1016/S2666-5247(23)00320-8
Cai, Yang, Liu, Chen, Shu et al., Experimental treatment with favipiravir for COVID-19: an open-label control study, Engineering (Beijing), doi:10.1093/bioinformatics/btp324
Cevik, Tate, Lloyd, Maraolo, Schafers et al., SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis, Lancet Microbe, doi:10.1093/bioinformatics/btp324
Cheema, Ali, Shahid, Ghafoor, Rehman et al., Efficacy and safety of favipiravir for the treatment of COVID-19 outpatients: a systematic review and metaanalysis of randomized controlled trials, Am J Ther, doi:10.1097/MJT.0000000000001649
Chen, Zhang, Huang, Yin, Cheng et al., Favipiravir versus Arbidol for COVID-19: a randomized clinical trial, Medrxiv, doi:10.1093/bioinformatics/btp324
Chen, Zhang, Huang, Yin, Cheng et al., Favipiravir versus arbidol for clinical recovery rate in moderate and severe adult COVID-19 patients: a prospective, multicenter, open-label, randomized controlled clinical trial, Front Pharmacol, doi:10.1093/bioinformatics/btp324
Diseases, Treatment Guidelines for COVID-19, doi:10.1093/bioinformatics/btp324
Gavenčiak, Monrad, Leech, Sharma, Mindermann et al., Seasonal variation in SARS-CoV-2 transmission in temperate climates: a bayesian modelling study in 143 European regions, PLoS Comput Biol, doi:10.1093/bioinformatics/btp324
Harvey, Carabelli, Jackson, Gupta, Thomson et al., SARS-CoV-2 variants, spike mutations and immune escape, Nat Rev Microbiol, doi:10.1093/bioinformatics/btp324
Hayden, Lenk, Stonis, Oldham-Creamer, Kang et al., Favipiravir treatment of uncomplicated influenza in adults: results of two phase 3, randomized, double-blind, placebo-controlled trials, J Infect Dis, doi:10.1016/j.ijid.2020.11.142
Holubar, Subramanian, Purington, Hedlin, Bunning et al., Favipiravir for treatment of outpatients with asymptomatic or uncomplicated coronavirus disease 2019: a double-blind, randomized, placebocontrolled, phase 2 trial, Clin Infect Dis, doi:10.1093/cid/ciac312
Illingworth, Guerra-Assuncao, Gregg, Charles, Pang et al., Genetic consequences of effective and suboptimal dosing with mutagenic drugs in a hamster model of SARS-CoV-2 infection, Virus Evol, doi:10.1093/bioinformatics/btp324
Illingworth, SAMFIRE: multi-locus variant calling for timeresolved sequence data, Bioinformatics, doi:10.1093/bioinformatics/btw205
Li, Durbin, Fast and accurate short read alignment with Burrows-Wheeler transform, Bioinformatics, doi:10.1093/bioinformatics/btp324
Marshall, Murthy, Diaz, Adhikari, Angus et al., A minimal common outcome measure set for COVID-19 clinical research, Lancet Infect Dis, doi:10.1093/bioinformatics/btp324
Ruiz-Rodriguez, Francés-Gómez, Chiner-Oms, López, Jiménez-Serrano et al., Evolutionary and phenotypic characterization of two spike mutations in European lineage 20E of SARS-CoV-2, mBio, doi:10.1128/mBio.02315-21
Sanderson, Hisner, Donovan-Banfield, Hartman, Løchen et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6
Shannon, Selisko, Le, Huchting, Touret et al., Rapid incorporation of Favipiravir by the fast and permissive viral RNA polymerase complex results in SARS-CoV-2 lethal mutagenesis, Nat Commun, doi:10.1093/cid/ciac312
Sissoko, Laouenan, Folkesson, Lebing, Beavogui et al., Experimental treatment with favipiravir for Ebola virus disease (the JIKI trial): a historically controlled, single-arm proof-of-concept trial in Guinea, PLoS Med, doi:10.1093/bioinformatics/btp324
Smee, Hurst, Egawa, Takahashi, Kadota et al., Intracellular metabolism of favipiravir (T-705) in uninfected and influenza A (H5N1) virus-infected cells, J Antimicrob Chemother, doi:10.1093/bioinformatics/btp324
Smith, Haigh, The hitch-hiking effect of a favourable gene, Antimicrobial Agents and Chemotherapy Month
Tsuzuki, Hayakawa, Doi, Shinozaki, Uemura et al., Effectiveness of favipiravir on nonsevere, earlystage COVID-19 in Japan: a large observational study using the COVID-19 registry Japan, Infect Dis Ther, doi:10.1007/s40121-022-00617-9
Udwadia, Singh, Barkate, Patil, Rangwala et al., Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int J Infect Dis, doi:10.1016/j.ijid.2020.11.142
Vaezi, Salmasi, Soltaninejad, Salahi, Javanmard et al., Favipiravir in the treatment of outpatient COVID-19: a multicenter, randomized, triple-blind, placebo-controlled clinical trial, Adv Respir Med, doi:10.3390/arm91010004
Vo, La, Wu, Strymish, Ronan et al., Factors associated with severe COVID-19 among vaccinated adults treated in US veterans affairs hospitals, JAMA Netw Open, doi:10.1093/bioinformatics/btp324
Watson, Barnsley, Toor, Hogan, Winskill et al., Global impact of the first year of COVID-19 vaccination: a mathematical modelling study, Lancet Infect Dis, doi:10.1093/bioinformatics/btp324
DOI record:
{
"DOI": "10.1128/aac.00054-25",
"ISSN": [
"0066-4804",
"1098-6596"
],
"URL": "http://dx.doi.org/10.1128/aac.00054-25",
"abstract": "<jats:title>ABSTRACT</jats:title>\n <jats:sec>\n <jats:title/>\n <jats:p>\n Early community treatment of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection may reduce severe coronavirus disease (COVID-19) incidence. We evaluated clinical effectiveness, safety, and SARS-CoV-2 mutagenicity of favipiravir, an oral viral RNA polymerase inhibitor. We performed an open-label, community-based, randomized Phase III trial, recruiting non-hospitalized adults with mild COVID-19 (WHO ordinal severity score [OSS] ≤ 3). Positive cases were invited to web-based self-screening within 24 h using public health data. Exclusion criteria included symptoms for >7 days, pregnancy/breastfeeding, severe renal/liver disease, gout, and licensed antiviral eligibility. Participants were randomized 1:1 to 10 days favipiravir (Day 1: 3,600 mg; days 2-10: 1,600 mg) or no additional treatment. The primary endpoint was worst recorded OSS up to and including Day 15 (intention-to-treat). The target recruitment was 302. Secondary endpoints included adverse event (AE) rate to Day 60, time-to-viral clearance (TTVC), time-to-symptom resolution (TTSR), and SARS-CoV-2 sequencing variant rate (≥5% frequency) at Day 15 (registration ISRCTN: 31062548; EudraCT: 2020-001904-41). A total of 68,788 adults were invited, and 302 (0.4%) were subsequently randomized between December 2020 and July 2022 (favipiravir [\n <jats:italic toggle=\"yes\">n</jats:italic>\n = 152]: standard care [\n <jats:italic toggle=\"yes\">n</jats:italic>\n = 150]). Mean (SD) age was 47.2 (13.2), and 230/302 (76%) were vaccinated. Severe outcomes were infrequent, with no intensive care unit admissions/deaths. There was no difference in the primary endpoint: odds ratio 1.18 (95% confidence interval [CI] 0.63–2.20), TTSR (HR 1.03 [95% CI 0.81–1.31]), or TTVC (HR 1.13 [95% CI 0.65–1.97]). Favipiravir was well tolerated with few AEs but was associated with increased variant frequency, including C-to-U mutations. Community administration of favipiravir for mild COVID-19 was not associated with clinical benefits or safety concerns but was associated with SARS-CoV-2 mutagenicity.\n </jats:p>\n <jats:sec>\n <jats:title>CLINICAL TRIALS</jats:title>\n <jats:p>\n This study is registered with ISRCTN as\n <jats:related-object xmlns:xlink=\"http://www.w3.org/1999/xlink\" document-id=\"31062548\" document-id-type=\"clinical-trial-number\" id=\"RO1\" source-id=\"ISRCTN\" source-id-type=\"registry-name\" source-type=\"clinical-trials-registry\" xlink:href=\"https://www.isrctn.com/ISRCTN31062548?q=31062548&filters=&sort=&offset=1&totalResults=1&page=1&pageSize=10\">31062548</jats:related-object>\n and with EU-CTR as\n <jats:related-object xmlns:xlink=\"http://www.w3.org/1999/xlink\" document-id=\"2020-001904-41\" document-id-type=\"clinical-trial-number\" id=\"RO2\" source-id=\"EU-CTR\" source-id-type=\"registry-name\" source-type=\"clinical-trials-registry\" xlink:href=\"https://www.clinicaltrialsregister.eu/ctr-search/search?query=2020-001904-41\">2020-001904-41</jats:related-object>\n .\n </jats:p>\n </jats:sec>\n </jats:sec>",
"alternative-id": [
"10.1128/aac.00054-25"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2025-01-18"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2025-05-07"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2025-06-24"
}
],
"author": [
{
"ORCID": "https://orcid.org/0009-0005-5658-5102",
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04y0x0x35",
"id-type": "ROR"
}
],
"name": "Department of Respiratory Medicine, Queen Elizabeth University Hospital",
"place": [
"Glasgow, United Kingdom"
]
},
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/00vtgdb53",
"id-type": "ROR"
}
],
"name": "School of Cancer Sciences, University of Glasgow",
"place": [
"Glasgow, United Kingdom"
]
}
],
"authenticated-orcid": true,
"family": "Tate",
"given": "Matthew",
"sequence": "first"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/00vtgdb53",
"id-type": "ROR"
}
],
"name": "MRC University of Glasgow Centre for Virus Research, University of Glasgow",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Illingworth",
"given": "Christopher J. R.",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04y0x0x35",
"id-type": "ROR"
}
],
"name": "Department of Respiratory Medicine, Queen Elizabeth University Hospital",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "MacGregor",
"given": "Gordon",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/03qxptw71",
"id-type": "ROR"
}
],
"name": "Cancer Research UK-Glasgow Clinical Trials Unit",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Cunningham",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/03qxptw71",
"id-type": "ROR"
}
],
"name": "Cancer Research UK-Glasgow Clinical Trials Unit",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Divers",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/03qxptw71",
"id-type": "ROR"
}
],
"name": "Cancer Research UK-Glasgow Clinical Trials Unit",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "McCartney",
"given": "Elaine",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/03qxptw71",
"id-type": "ROR"
}
],
"name": "Cancer Research UK-Glasgow Clinical Trials Unit",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Paterson",
"given": "Lucy",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/03qxptw71",
"id-type": "ROR"
}
],
"name": "Cancer Research UK-Glasgow Clinical Trials Unit",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Kelly",
"given": "Caroline",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/03qxptw71",
"id-type": "ROR"
}
],
"name": "Cancer Research UK-Glasgow Clinical Trials Unit",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Shaw",
"given": "Ann",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04y0x0x35",
"id-type": "ROR"
}
],
"name": "Glasgow Clinical Research Facility, Queen Elizabeth University Hospital",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Perkins",
"given": "Jonathan S.",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04y0x0x35",
"id-type": "ROR"
}
],
"name": "Glasgow Clinical Research Facility, Queen Elizabeth University Hospital",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Silva",
"given": "Vanessa",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04y0x0x35",
"id-type": "ROR"
}
],
"name": "Glasgow Clinical Research Facility, Queen Elizabeth University Hospital",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Holland",
"given": "Poppy",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04y0x0x35",
"id-type": "ROR"
}
],
"name": "Glasgow Clinical Research Facility, Queen Elizabeth University Hospital",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Dalton",
"given": "Carol",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04y0x0x35",
"id-type": "ROR"
}
],
"name": "Glasgow Clinical Research Facility, Queen Elizabeth University Hospital",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Carmichael",
"given": "Samantha",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04y0x0x35",
"id-type": "ROR"
}
],
"name": "Glasgow Clinical Research Facility, Queen Elizabeth University Hospital",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Douglas",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04y0x0x35",
"id-type": "ROR"
}
],
"name": "Glasgow Clinical Research Facility, Queen Elizabeth University Hospital",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Surtees",
"given": "Pamela",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/00vtgdb53",
"id-type": "ROR"
}
],
"name": "MRC University of Glasgow Centre for Virus Research, University of Glasgow",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Scott",
"given": "Janet T.",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/00vtgdb53",
"id-type": "ROR"
}
],
"name": "BHF Glasgow Cardiovascular Research Centre, University of Glasgow",
"place": [
"Glasgow, United Kingdom"
]
},
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/00vtgdb53",
"id-type": "ROR"
}
],
"name": "School of Cardiovascular and Metabolic Health, University of Glasgow",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Berry",
"given": "Colin",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/00vtgdb53",
"id-type": "ROR"
}
],
"name": "MRC University of Glasgow Centre for Virus Research, University of Glasgow",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Vattipally",
"given": "Sreenu",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/00vtgdb53",
"id-type": "ROR"
}
],
"name": "MRC University of Glasgow Centre for Virus Research, University of Glasgow",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Da Silva Filipe",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/00vtgdb53",
"id-type": "ROR"
}
],
"name": "MRC University of Glasgow Centre for Virus Research, University of Glasgow",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Tong",
"given": "Lily",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/00bjck208",
"id-type": "ROR"
}
],
"name": "West of Scotland Specialist Virology Centre, Glasgow Royal Infirmary",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Gunson",
"given": "Rory",
"sequence": "additional"
},
{
"affiliation": [],
"name": "GETAFIX trial co-investigators",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/00vtgdb53",
"id-type": "ROR"
}
],
"name": "School of Infection and Immunity, University of Glasgow",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "McInnes",
"given": "Iain B.",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/00vtgdb53",
"id-type": "ROR"
}
],
"name": "School of Cancer Sciences, University of Glasgow",
"place": [
"Glasgow, United Kingdom"
]
},
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/03qxptw71",
"id-type": "ROR"
}
],
"name": "Cancer Research UK-Glasgow Clinical Trials Unit",
"place": [
"Glasgow, United Kingdom"
]
}
],
"family": "Jones",
"given": "Robert",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1482-0889",
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/00vtgdb53",
"id-type": "ROR"
}
],
"name": "MRC University of Glasgow Centre for Virus Research, University of Glasgow",
"place": [
"Glasgow, United Kingdom"
]
},
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/00vtgdb53",
"id-type": "ROR"
}
],
"name": "School of Infection and Immunity, University of Glasgow",
"place": [
"Glasgow, United Kingdom"
]
}
],
"authenticated-orcid": true,
"family": "Thomson",
"given": "Emma",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2972-6641",
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04y0x0x35",
"id-type": "ROR"
}
],
"name": "Department of Respiratory Medicine, Queen Elizabeth University Hospital",
"place": [
"Glasgow, United Kingdom"
]
},
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/00vtgdb53",
"id-type": "ROR"
}
],
"name": "School of Cancer Sciences, University of Glasgow",
"place": [
"Glasgow, United Kingdom"
]
},
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/03pv69j64",
"id-type": "ROR"
}
],
"name": "Cancer Research UK Scotland Institute",
"place": [
"Glasgow, United Kingdom"
]
}
],
"authenticated-orcid": false,
"family": "Blyth",
"given": "Kevin G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rooney",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McGarry",
"given": "Erin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meharry",
"given": "Rosemary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Devers",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Akyol",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pearson",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nichol",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Casey",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McGettrick",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brock",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Nathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McCall",
"given": "Rebecca",
"sequence": "additional"
}
],
"container-title": "Antimicrobial Agents and Chemotherapy",
"container-title-short": "Antimicrob Agents Chemother",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.asm.org"
]
},
"created": {
"date-parts": [
[
2025,
6,
24
]
],
"date-time": "2025-06-24T13:00:06Z",
"timestamp": 1750770006000
},
"deposited": {
"date-parts": [
[
2025,
6,
24
]
],
"date-time": "2025-06-24T13:00:07Z",
"timestamp": 1750770007000
},
"editor": [
{
"affiliation": [],
"family": "Martinez",
"given": "Miguel Angel",
"sequence": "additional"
}
],
"funder": [
{
"DOI": "10.13039/100014589",
"award": [
"COV/GLA/20/03"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100014589",
"id-type": "DOI"
}
],
"name": "Chief Scientist Office, Scottish Government Health and Social Care Directorate"
}
],
"indexed": {
"date-parts": [
[
2025,
6,
24
]
],
"date-time": "2025-06-24T13:40:03Z",
"timestamp": 1750772403118,
"version": "3.41.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
6,
24
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
6,
24
]
],
"date-time": "2025-06-24T00:00:00Z",
"timestamp": 1750723200000
}
},
{
"URL": "https://journals.asm.org/non-commercial-tdm-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
6,
24
]
],
"date-time": "2025-06-24T00:00:00Z",
"timestamp": 1750723200000
}
}
],
"link": [
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/aac.00054-25",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/aac.00054-25",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "235",
"original-title": [],
"prefix": "10.1128",
"published": {
"date-parts": [
[
2025,
6,
24
]
]
},
"published-online": {
"date-parts": [
[
2025,
6,
24
]
]
},
"publisher": "American Society for Microbiology",
"reference": [
{
"key": "e_1_3_4_2_2",
"unstructured": "World Health Organisation. 2025. WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int"
},
{
"DOI": "10.1016/S1473-3099(22)00320-6",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_3_2"
},
{
"DOI": "10.1001/jamanetworkopen.2022.40037",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_4_2"
},
{
"DOI": "10.1371/journal.pcbi.1010435",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_5_2"
},
{
"DOI": "10.1038/s41579-021-00573-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_6_2"
},
{
"DOI": "10.1016/S2666-5247(20)30172-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_7_2"
},
{
"DOI": "10.1093/jac/dkp274",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_8_2"
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_9_2"
},
{
"DOI": "10.1101/2020.03.17.20037432",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_10_2",
"unstructured": "Chen C Zhang Y Huang J Yin P Cheng Z Wu J Chen S Zhang Y Chen B Lu M Luo Y Ju L Zhang J Wang X. 2020. Favipiravir versus Arbidol for COVID-19: a randomized clinical trial. Medrxiv. doi:10.1101/2020.03.17.20037432"
},
{
"DOI": "10.1371/journal.pmed.1001967",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_11_2"
},
{
"DOI": "10.3389/fphar.2021.683296",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_12_2"
},
{
"key": "e_1_3_4_13_2",
"unstructured": "Diseases JA for I. 2020. Treatment Guidelines for COVID-19. Available from: https://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_drug_200514.pdf"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_14_2"
},
{
"DOI": "10.1093/ve/veae001",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_15_2"
},
{
"key": "e_1_3_4_16_2",
"unstructured": "GitHub. 2025. FelixKrueger/TrimGalore: a wrapper around cutadapt and FastQC to consistently apply adapter and quality trimming to FastQ Files with extra functionality for RRBS data. https://github.com/FelixKrueger/TrimGalore."
},
{
"DOI": "10.1093/bioinformatics/btp324",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_17_2"
},
{
"DOI": "10.1093/bioinformatics/btw205",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_18_2"
},
{
"DOI": "10.1016/S2666-5247(23)00320-8",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_19_2"
},
{
"DOI": "10.1128/mBio.02315-21",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_20_2"
},
{
"DOI": "10.3390/arm91010004",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_21_2"
},
{
"DOI": "10.1097/MJT.0000000000001649",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_22_2"
},
{
"DOI": "10.1007/s40121-022-00617-9",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_23_2"
},
{
"DOI": "10.1093/infdis/jiac135",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_24_2"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_25_2"
},
{
"DOI": "10.1038/s41586-023-06649-6",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_26_2"
},
{
"DOI": "10.1038/s41467-020-18463-z",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_27_2"
},
{
"DOI": "10.1093/cid/ciac312",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_28_2"
},
{
"DOI": "10.1017/S0016672300014634",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_29_2"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.asm.org/doi/10.1128/aac.00054-25"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Clinical effectiveness, safety, and viral mutagenicity of oral favipiravir for COVID-19: results from a community-based, open-label, randomized Phase III trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1128/asmj-crossmark-policy-page"
}