Effectiveness of Favipiravir on Nonsevere, Early-Stage COVID-19 in Japan: A Large Observational Study Using the COVID-19 Registry Japan
et al., Infectious Diseases and Therapy, doi:10.1007/s40121-022-00617-9, Mar 2022
Retrospective database analysis of 7,654 hospitalized patients in Japan, showing no significant differences with favipiravir treatment. NCGM-G-003494-0.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments15.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
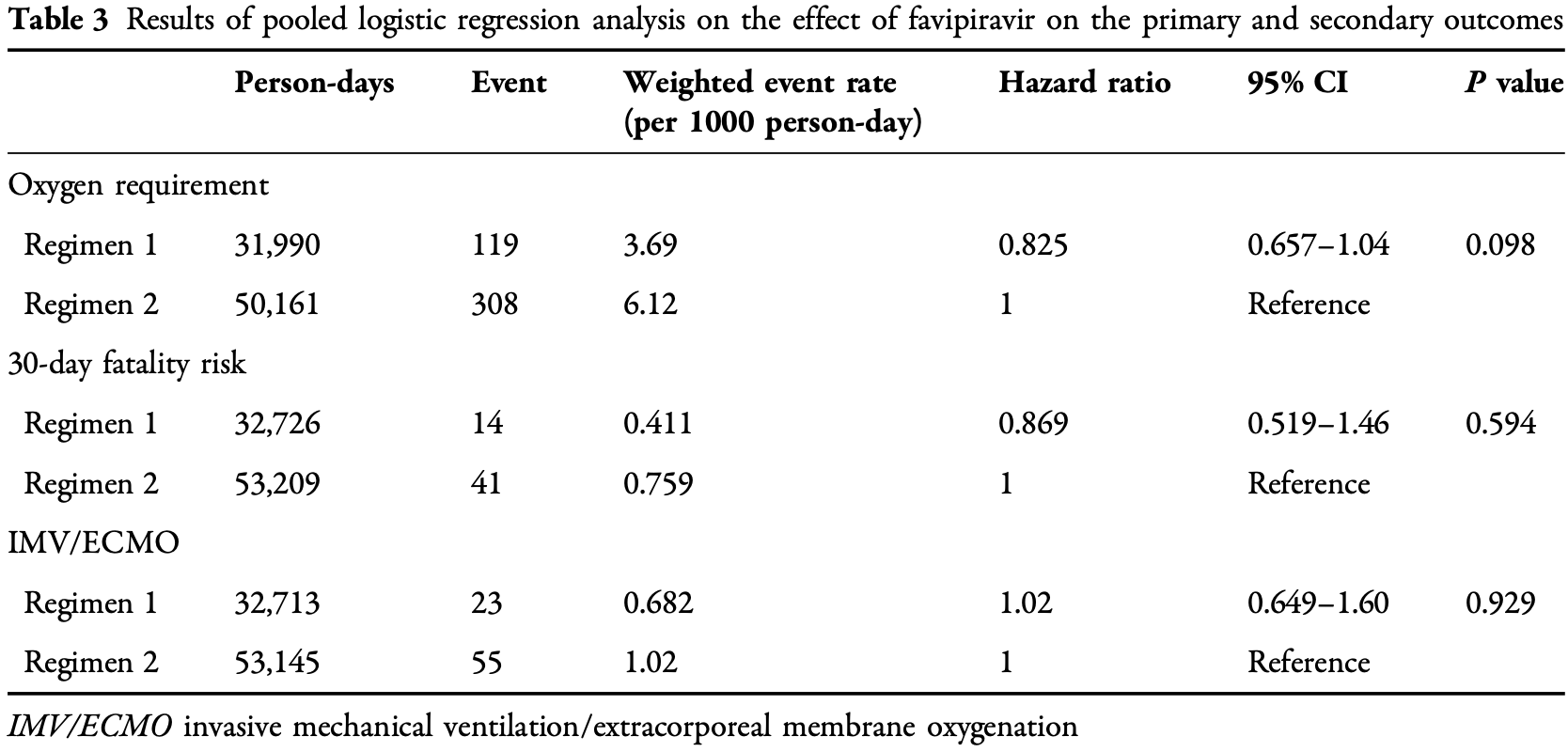
risk of death, 13.1% lower, HR 0.87, p = 0.59, treatment 2,532, control 5,122, adjusted per study, day 30.
|
|
risk of mechanical ventilation, 2.0% higher, HR 1.02, p = 0.93, treatment 2,532, control 5,122, adjusted per study, IMV/ECMO.
|
|
risk of progression, 17.5% lower, HR 0.82, p = 0.10, treatment 2,532, control 5,122, adjusted per study, oxygen requirement.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
13.
Cenikli et al., Does Favipiravir interact with DNA? Design of electrochemical DNA nanobiosensor to investigate the interaction between DNA and Favipiravir used in the treatment of COVID-19, Talanta, doi:10.1016/j.talanta.2025.128084.
Tsuzuki et al., 21 Mar 2022, retrospective, Japan, peer-reviewed, 21 authors, average treatment delay 4.0 days.
Effectiveness of Favipiravir on Nonsevere, Early-Stage COVID-19 in Japan: A Large Observational Study Using the COVID-19 Registry Japan
Infectious Diseases and Therapy, doi:10.1007/s40121-022-00617-9
Introduction: Several randomized controlled trials have compared the effectiveness of favipiravir with that of placebo. However, evidence regarding its effect on nonsevere, early-stage coronavirus disease 2019 (COVID-19) remains insufficient. Methods: We used the COVID-19 Registry Japan, a nationwide registry of inpatients with COVID-19, for evaluating the effectiveness of favipiravir on patients with nonsevere, earlystage COVID-19. Eligible patients, who did not need supplementary oxygen therapy at admission, were classified according to two regimens (starting favipiravir therapy within 4 days from admission vs. no favipiravir during hospitalization) and were then compared using a threestep method (cloning, censoring, and
Ethics Author Contributions. Shinya Tsuzuki, Kayoko Hayakawa, Yohei Doi, Wataru Sugiura, and Norio Ohmagari conceived the study. Tomohiro Shinozaki and Yukari Uemura designed analysis. Nobuaki Matsunaga, Mari Terada, Setsuko Suzuki, Yusuke Asai, Taro Shibata, Masahi Kondo, Kazuo Izumi, Masahiro Hojo, Tetsuya Mizoue, Kazuhisa Yokota, Fukumi Nakamura-Uchiyama, and Fumitake Saito collected the data. Shinya Tsuzuki, Kayoko Hayakawa, Yohei Doi, Tomohiro Shinozaki, Yukari Uemura, Sho Saito, and Gen Yamada analyzed and interpreted the data. Shinya Tsuzuki wrote the first draft, which was subsequently revised by all authors. All authors read and approved the final manuscript. Disclosures. Yohei Doi received scholarship donation from Shionogi & Co., Ltd., honorarium from Merck & Co., Shionogi & Co., Ltd., FUJIFILM Toyama Chemical Co., Ltd., and Teijin Pharma Limited, consultant fee from Shionogi & Co., Ltd., Meiji Seika Pharma Co., Ltd., Gilead Sciences, Inc., bioMe ´rieux S.A., GlaxoSmithKline plc, Merck & Co., and Chugai Pharmaceutical Co., Ltd. Shinya Tsuzuki, Kayoko Hayakawa, Tomohiro Shinozaki, Yukari Uemura, Nobuaki Matsunaga, Mari Terada, Setsuko Suzuki, Yusuke Asai, Gen Yamada, Sho Saito, Taro Shibata, Masashi Kondo, Kazuo Izumi, Masayuki Hojo, Tetsuya Mizoue, Kazuhisa Yokota, Fukumi Nakamura-Uchiyama, Fumitake Saito, Wataru Sugiura, Norio Ohmagari all have nothing to disclose. Compliance with Ethics Guidelines. This study was approved by the NCGM ethics..
References
Bai, Mu, Kargbo, Clinical and virological characteristics of Ebola virus disease patients treated with favipiravir (T-705)-Sierra Leone, 2014, Clin Infect Dis
Bosaeed, Alharbi, Favipiravir and hydroxychloroquine combination therapy in patients with moderate to severe COVID-19 (FACCT Trial): an open-label, multicenter, randomized, controlled trial, Infect Dis Ther
Bosaeed, Alharbi, Mahmoud, Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial, Clin Microbiol Infect, doi:10.1016/j.cmi.2021.12.026
Core, R: a language and environment for statistical computing
Cunningham, Vaduganathan, Claggett, Clinical outcomes in young US adults hospitalized with COVID-19, JAMA Intern Med
Delang, Abdelnabi, Neyts, Favipiravir as a potential countermeasure against neglected and emerging RNA viruses, Antivir Res
Doi, Hibino, Hase, A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19, Antimicrob Agents Chemother
Finberg, Ashraf, Julg, US201 study: a phase 2, randomized proof-of-concept trial of favipiravir for the treatment of COVID-19, Open Forum Infect Dis
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc Jpn Acad Ser B Phys Biol Sci
Harris, Taylor, Thielke, Payne, Gonzalez et al., Research electronic data capture (RED-Cap)-a metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform
Hashemian, Farhadi, Velayati, A review on favipiravir: the properties, function, and usefulness to treat COVID-19, Expert Rev Anti Infect Ther
Herna ´n, How to estimate the effect of treatment duration on survival outcomes using observational data, BMJ
Herna ´n, Sauer, Herna ´ndez-Dı ´az, Platt, Shrier, Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses, J Clin Epidemiol
Ito, Ohmagari, Mikami, Sugiura, Major ongoing clinical trials for COVID-19 treatment and studies currently being conducted or scheduled in Japan, Glob Health Med
Ivashchenko, Dmitriev, Vostokova, AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): interim results of a phase II/III multicenter randomized clinical trial, Clin Infect Dis
Lai, Chao, Hsueh, Clinical efficacy of antiviral agents against coronavirus disease 2019: a systematic review of randomized controlled trials, J Microbiol Immunol Infect
Lighter, Phillips, Hochman, Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission, Clin Infect Dis
Matsunaga, Hayakawa, Terada, Clinical epidemiology of hospitalized patients with COVID-19 in Japan: report of the COVID-19 Registry Japan, Clin Infect Dis
Petrilli, Jones, Yang, Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study, BMJ
Pilkington, Pepperrell, Hill, A review of the safety of favipiravir-a potential treatment in the COVID-19 pandemic?, J Virus Erad
Ritchie, Mathieu, ´s-Guirao, Coronavirus pandemic (COVID-19)
Robins, Finkelstein, Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests, Biometrics
Rosenbaum, Db, The central role of the propensity score in observational studies for causal effects, Biometrilca
Shinkai, Tsushima, Tanaka, Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, phase III clinical trial, Infect Dis Ther
Sissoko, Laouenan, Folkesson, Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in guinea, PLoS Med
Suissa, Dell'aniello, Time-related biases in pharmacoepidemiology, Pharmacoepidemiol Drug Saf
Tartof, Qian, Hong, Obesity and mortality among patients diagnosed with COVID-19: Infect Dis Ther results from an integrated health care organization, Ann Intern Med
Tsuzuki, Hayakawa, Uemura, Efficacy of remdesivir in hospitalised COVID-19 patients in Japan: a large observational study using the COVID-19 Registry Japan, doi:10.1101/2021.03.09.21253183v2
Udwadia, Singh, Barkate, Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int J Infect Dis
Williamson, Walker, Bhaskaran, Factors associated with COVID-19-related death using OpenSAFELY, Nature
Wu, Mcgoogan, Characteristics of and Important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention, JAMA
DOI record:
{
"DOI": "10.1007/s40121-022-00617-9",
"ISSN": [
"2193-8229",
"2193-6382"
],
"URL": "http://dx.doi.org/10.1007/s40121-022-00617-9",
"alternative-id": [
"617"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "24 January 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "23 February 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "21 March 2022"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-5732-846X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Tsuzuki",
"given": "Shinya",
"sequence": "first"
},
{
"affiliation": [],
"family": "Hayakawa",
"given": "Kayoko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Doi",
"given": "Yohei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shinozaki",
"given": "Tomohiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Uemura",
"given": "Yukari",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matsunaga",
"given": "Nobuaki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Terada",
"given": "Mari",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suzuki",
"given": "Setsuko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Asai",
"given": "Yusuke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yamada",
"given": "Gen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saito",
"given": "Sho",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shibata",
"given": "Taro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kondo",
"given": "Masashi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Izumi",
"given": "Kazuo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hojo",
"given": "Masayuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mizoue",
"given": "Tetsuya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yokota",
"given": "Kazuhisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nakamura-Uchiyama",
"given": "Fukumi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saito",
"given": "Fumitake",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sugiura",
"given": "Wataru",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ohmagari",
"given": "Norio",
"sequence": "additional"
}
],
"container-title": [
"Infectious Diseases and Therapy"
],
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
3,
21
]
],
"date-time": "2022-03-21T02:02:48Z",
"timestamp": 1647828168000
},
"deposited": {
"date-parts": [
[
2022,
3,
21
]
],
"date-time": "2022-03-21T02:14:01Z",
"timestamp": 1647828841000
},
"funder": [
{
"DOI": "10.13039/501100003478",
"award": [
"19HA1003"
],
"doi-asserted-by": "publisher",
"name": "Ministry of Health, Labour and Welfare"
}
],
"indexed": {
"date-parts": [
[
2022,
3,
21
]
],
"date-time": "2022-03-21T02:41:53Z",
"timestamp": 1647830513388
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "2193-8229"
},
{
"type": "electronic",
"value": "2193-6382"
}
],
"issued": {
"date-parts": [
[
2022,
3,
21
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
21
]
],
"date-time": "2022-03-21T00:00:00Z",
"timestamp": 1647820800000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
21
]
],
"date-time": "2022-03-21T00:00:00Z",
"timestamp": 1647820800000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-022-00617-9.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s40121-022-00617-9/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-022-00617-9.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2022,
3,
21
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
21
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.2183/pjab.93.027",
"author": "Y Furuta",
"doi-asserted-by": "publisher",
"first-page": "449",
"journal-title": "Proc Jpn Acad Ser B Phys Biol Sci",
"key": "617_CR1",
"unstructured": "Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:449–63.",
"volume": "93",
"year": "2017"
},
{
"key": "617_CR2",
"unstructured": "Information of Avigan Tablet in relation to Covid-19 | FUJIFILM Toyama Chemical Co.. https://www.fujifilm.com/fftc/en/avigan. Accessed 8 Nov 2021."
},
{
"DOI": "10.1093/cid/ciw571",
"author": "C-Q Bai",
"doi-asserted-by": "publisher",
"first-page": "1288",
"journal-title": "Clin Infect Dis",
"key": "617_CR3",
"unstructured": "Bai C-Q, Mu J-S, Kargbo D, et al. Clinical and virological characteristics of Ebola virus disease patients treated with favipiravir (T-705)-Sierra Leone, 2014. Clin Infect Dis. 2016;63:1288–94.",
"volume": "63",
"year": "2016"
},
{
"DOI": "10.1371/journal.pmed.1001967",
"author": "D Sissoko",
"doi-asserted-by": "publisher",
"first-page": "e1001967",
"journal-title": "PLoS Med",
"key": "617_CR4",
"unstructured": "Sissoko D, Laouenan C, Folkesson E, et al. Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in guinea. PLoS Med. 2016;13:e1001967.",
"volume": "13",
"year": "2016"
},
{
"DOI": "10.1128/AAC.01897-20",
"author": "Y Doi",
"doi-asserted-by": "publisher",
"first-page": "e01897-20",
"journal-title": "Antimicrob Agents Chemother",
"key": "617_CR5",
"unstructured": "Doi Y, Hibino M, Hase R, et al. A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19. Antimicrob Agents Chemother. 2020;64:e01897-20.",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.35772/ghm.2020.01034",
"author": "K Ito",
"doi-asserted-by": "publisher",
"first-page": "96",
"journal-title": "Glob Health Med",
"key": "617_CR6",
"unstructured": "Ito K, Ohmagari N, Mikami A, Sugiura W. Major ongoing clinical trials for COVID-19 treatment and studies currently being conducted or scheduled in Japan. Glob Health Med. 2020;2:96–101.",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1176",
"author": "AA Ivashchenko",
"doi-asserted-by": "publisher",
"first-page": "531",
"journal-title": "Clin Infect Dis",
"key": "617_CR7",
"unstructured": "Ivashchenko AA, Dmitriev KA, Vostokova NV, et al. AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): interim results of a phase II/III multicenter randomized clinical trial. Clin Infect Dis. 2021;73:531–4.",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1007/s40121-021-00517-4",
"author": "M Shinkai",
"doi-asserted-by": "publisher",
"first-page": "2489",
"journal-title": "Infect Dis Ther",
"key": "617_CR8",
"unstructured": "Shinkai M, Tsushima K, Tanaka S, et al. Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, phase III clinical trial. Infect Dis Ther. 2021;10:2489–509.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"author": "ZF Udwadia",
"doi-asserted-by": "publisher",
"first-page": "62",
"journal-title": "Int J Infect Dis",
"key": "617_CR9",
"unstructured": "Udwadia ZF, Singh P, Barkate H, et al. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis. 2021;103:62–71.",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1007/s40121-021-00496-6",
"author": "M Bosaeed",
"doi-asserted-by": "publisher",
"first-page": "2291",
"journal-title": "Infect Dis Ther",
"key": "617_CR10",
"unstructured": "Bosaeed M, Mahmoud E, Alharbi A, et al. Favipiravir and hydroxychloroquine combination therapy in patients with moderate to severe COVID-19 (FACCT Trial): an open-label, multicenter, randomized, controlled trial. Infect Dis Ther. 2021;10:2291–307.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1016/j.jmii.2021.05.011",
"author": "C-C Lai",
"doi-asserted-by": "publisher",
"first-page": "767",
"journal-title": "J Microbiol Immunol Infect",
"key": "617_CR11",
"unstructured": "Lai C-C, Chao C-M, Hsueh P-R. Clinical efficacy of antiviral agents against coronavirus disease 2019: a systematic review of randomized controlled trials. J Microbiol Immunol Infect. 2021;54:767–75.",
"volume": "54",
"year": "2021"
},
{
"DOI": "10.1016/j.cmi.2021.12.026",
"doi-asserted-by": "publisher",
"key": "617_CR12",
"unstructured": "Bosaeed M, Alharbi A, Mahmoud E, et al. Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial. Clin Microbiol Infect. 2022. https://doi.org/10.1016/j.cmi.2021.12.026."
},
{
"DOI": "10.1093/ofid/ofab563",
"author": "RW Finberg",
"doi-asserted-by": "publisher",
"first-page": "ofab563",
"journal-title": "Open Forum Infect Dis",
"key": "617_CR13",
"unstructured": "Finberg RW, Ashraf M, Julg B, et al. US201 study: a phase 2, randomized proof-of-concept trial of favipiravir for the treatment of COVID-19. Open Forum Infect Dis. 2021;8:ofab563.",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1470",
"author": "N Matsunaga",
"doi-asserted-by": "publisher",
"first-page": "e3677",
"journal-title": "Clin Infect Dis",
"key": "617_CR14",
"unstructured": "Matsunaga N, Hayakawa K, Terada M, et al. Clinical epidemiology of hospitalized patients with COVID-19 in Japan: report of the COVID-19 Registry Japan. Clin Infect Dis. 2020;73:e3677–89.",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1016/j.jbi.2008.08.010",
"author": "PA Harris",
"doi-asserted-by": "publisher",
"first-page": "377",
"journal-title": "J Biomed Inform",
"key": "617_CR15",
"unstructured": "Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.",
"volume": "42",
"year": "2009"
},
{
"key": "617_CR16",
"unstructured": "Ministry of Health, Labour and Welfare. Clinical Guideline for COVID-19. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000121431_00111.html. Accessed 12 Aug 2021."
},
{
"DOI": "10.1002/pds.5083",
"author": "S Suissa",
"doi-asserted-by": "publisher",
"first-page": "1101",
"journal-title": "Pharmacoepidemiol Drug Saf",
"key": "617_CR17",
"unstructured": "Suissa S, Dell’Aniello S. Time-related biases in pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2020;29:1101–10.",
"volume": "29",
"year": "2020"
},
{
"DOI": "10.1016/j.jclinepi.2016.04.014",
"author": "MA Hernán",
"doi-asserted-by": "publisher",
"first-page": "70",
"journal-title": "J Clin Epidemiol",
"key": "617_CR18",
"unstructured": "Hernán MA, Sauer BC, Hernández-Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70–5.",
"volume": "79",
"year": "2016"
},
{
"DOI": "10.1101/2021.03.09.21253183v2",
"author": "S Tsuzuki",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "617_CR19",
"unstructured": "Tsuzuki S, Hayakawa K, Uemura Y, et al. Efficacy of remdesivir in hospitalised COVID-19 patients in Japan: a large observational study using the COVID-19 Registry Japan. medRxiv. 2021. https://doi.org/10.1101/2021.03.09.21253183v2.",
"year": "2021"
},
{
"DOI": "10.1136/bmj.k182",
"author": "MA Hernán",
"doi-asserted-by": "publisher",
"first-page": "k182",
"journal-title": "BMJ",
"key": "617_CR20",
"unstructured": "Hernán MA. How to estimate the effect of treatment duration on survival outcomes using observational data. BMJ. 2018;360:k182.",
"volume": "360",
"year": "2018"
},
{
"DOI": "10.1111/j.0006-341X.2000.00779.x",
"author": "JM Robins",
"doi-asserted-by": "publisher",
"first-page": "779",
"journal-title": "Biometrics",
"key": "617_CR21",
"unstructured": "Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56:779–88.",
"volume": "56",
"year": "2000"
},
{
"key": "617_CR22",
"unstructured": "The National Health Service England. National Early Warning Score (NEWS). https://www.england.nhs.uk/ourwork/clinical-policy/sepsis/nationalearlywarningscore/. Accessed 28 Jan 2021."
},
{
"DOI": "10.1001/jama.2020.2648",
"author": "Z Wu",
"doi-asserted-by": "publisher",
"first-page": "1239",
"journal-title": "JAMA",
"key": "617_CR23",
"unstructured": "Wu Z, McGoogan JM. Characteristics of and Important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1966",
"author": "CM Petrilli",
"doi-asserted-by": "publisher",
"first-page": "m1966",
"journal-title": "BMJ",
"key": "617_CR24",
"unstructured": "Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966.",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2521-4",
"author": "EJ Williamson",
"doi-asserted-by": "publisher",
"first-page": "430",
"journal-title": "Nature",
"key": "617_CR25",
"unstructured": "Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–6.",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.5313",
"author": "JW Cunningham",
"doi-asserted-by": "publisher",
"first-page": "379",
"journal-title": "JAMA Intern Med",
"key": "617_CR26",
"unstructured": "Cunningham JW, Vaduganathan M, Claggett BL, et al. Clinical outcomes in young US adults hospitalized with COVID-19. JAMA Intern Med. 2020;181:379–81.",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa415",
"author": "J Lighter",
"doi-asserted-by": "publisher",
"first-page": "896",
"journal-title": "Clin Infect Dis",
"key": "617_CR27",
"unstructured": "Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71:896–7.",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.7326/M20-3742",
"author": "SY Tartof",
"doi-asserted-by": "publisher",
"first-page": "773",
"journal-title": "Ann Intern Med",
"key": "617_CR28",
"unstructured": "Tartof SY, Qian L, Hong V, et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173:773–81.",
"volume": "173",
"year": "2020"
},
{
"key": "617_CR29",
"unstructured": "R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018."
},
{
"key": "617_CR30",
"unstructured": "Appili Therapeutics provides update on phase 3 PRESECO clinical trial evaluating Avigan®/Reeqonus™. Appili—LIVE. https://www.appilitherapeutics.com/newsfeed/Appili-Therapeutics-Provides-Update-on-Phase-3-PRESECO-Clinical-Trial-Evaluating-Avigan%C2%AE%2FReeqonus%E2%84%A2. Accessed 17 Nov 2021."
},
{
"key": "617_CR31",
"unstructured": "National Institutes of Health. Information on COVID-19 treatment, prevention and research. COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/. Accessed 27 Jan 2021."
},
{
"key": "617_CR32",
"unstructured": "World Health Organization. Therapeutics and COVID-19: living guideline. https://www.who.int/publications-detail-redirect/therapeutics-and-covid-19-living-guideline. Accessed 27 Jan 2021."
},
{
"key": "617_CR33",
"unstructured": "CDC. Coronavirus disease 2019 (COVID-19). Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/coronavirus/2019-ncov/index.html. Accessed 12 Aug 2021."
},
{
"key": "617_CR34",
"unstructured": "Prime Minister’s Office of Japan. About novel coronavirus vaccine. https://www.kantei.go.jp/jp/headline/kansensho/vaccine.html. Accessed 18 Nov 2021."
},
{
"key": "617_CR35",
"unstructured": "Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our world in data. 2020. https://ourworldindata.org/covid-vaccinations. Accessed 18 Nov 2021."
},
{
"DOI": "10.1016/j.antiviral.2018.03.003",
"author": "L Delang",
"doi-asserted-by": "publisher",
"first-page": "85",
"journal-title": "Antivir Res",
"key": "617_CR36",
"unstructured": "Delang L, Abdelnabi R, Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antivir Res. 2018;153:85–94.",
"volume": "153",
"year": "2018"
},
{
"DOI": "10.1016/S2055-6640(20)30016-9",
"author": "V Pilkington",
"doi-asserted-by": "publisher",
"first-page": "45",
"journal-title": "J Virus Erad",
"key": "617_CR37",
"unstructured": "Pilkington V, Pepperrell T, Hill A. A review of the safety of favipiravir—a potential treatment in the COVID-19 pandemic? J Virus Erad. 2020;6:45–51.",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1080/14787210.2021.1866545",
"author": "SM Hashemian",
"doi-asserted-by": "publisher",
"first-page": "1029",
"journal-title": "Expert Rev Anti Infect Ther",
"key": "617_CR38",
"unstructured": "Hashemian SM, Farhadi T, Velayati AA. A review on favipiravir: the properties, function, and usefulness to treat COVID-19. Expert Rev Anti Infect Ther. 2021;19:1029–37.",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1093/biomet/70.1.41",
"author": "PR Rosenbaum",
"doi-asserted-by": "publisher",
"first-page": "41",
"journal-title": "Biometrilca",
"key": "617_CR39",
"unstructured": "Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrilca. 1983;70:41–55.",
"volume": "70",
"year": "1983"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s40121-022-00617-9"
}
},
"score": 1,
"short-container-title": [
"Infect Dis Ther"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": [
"Effectiveness of Favipiravir on Nonsevere, Early-Stage COVID-19 in Japan: A Large Observational Study Using the COVID-19 Registry Japan"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}