Favipiravir for treatment of outpatients with asymptomatic or uncomplicated COVID-19: a double-blind randomized, placebo-controlled, phase 2 trial
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciac312, Nov 2021 (preprint)
Small RCT 116 mITT patients in the USA, 59 treated with favipiravir, showing no significant differences with treatment.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments15.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
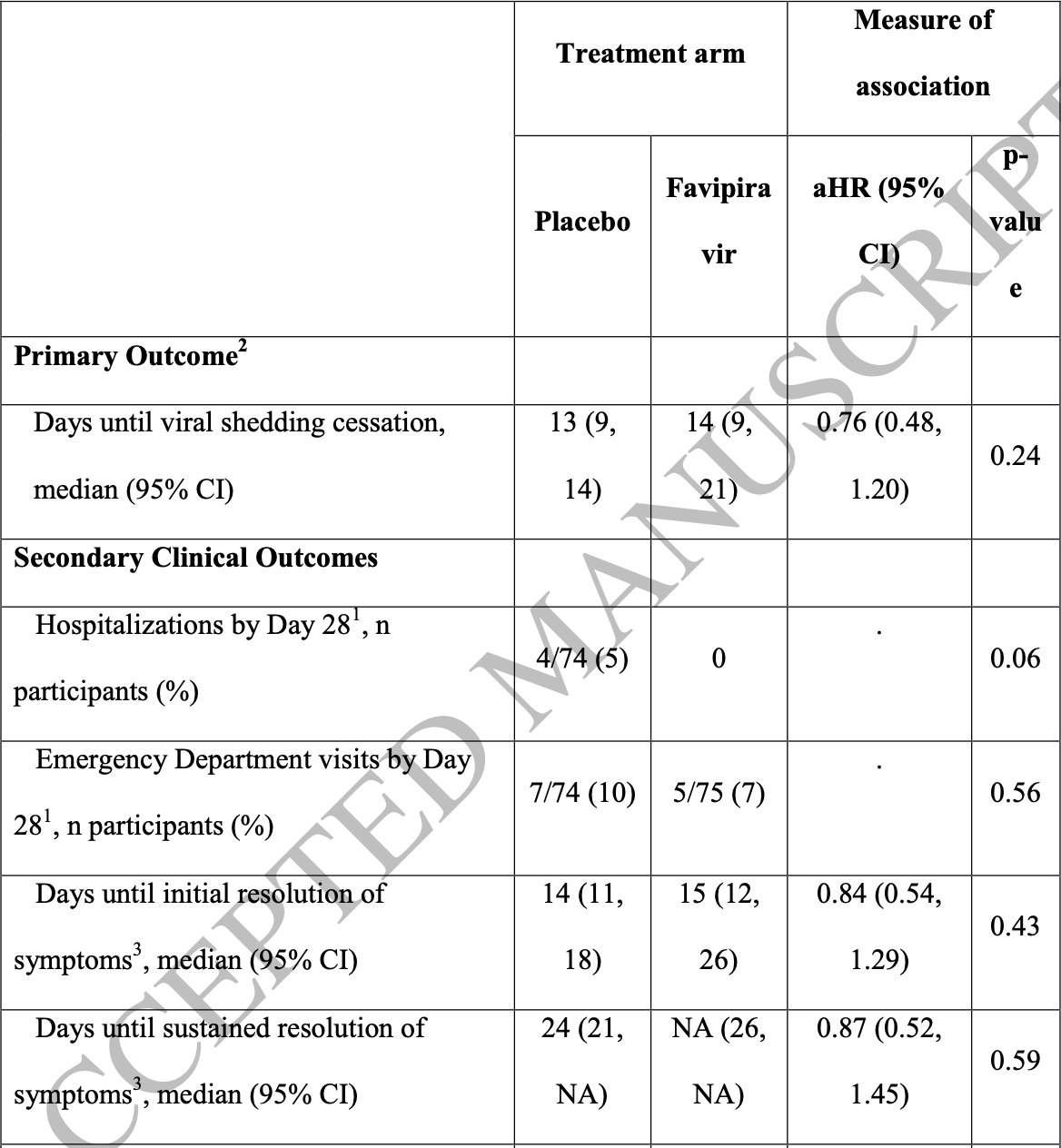
risk of hospitalization, 89.0% lower, RR 0.11, p = 0.06, treatment 0 of 75 (0.0%), control 4 of 74 (5.4%), NNT 18, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of ER visit, 29.5% lower, RR 0.70, p = 0.56, treatment 5 of 75 (6.7%), control 7 of 74 (9.5%), NNT 36.
|
|
risk of no recovery, 19.0% higher, RR 1.19, p = 0.43, treatment 65, control 70, inverted to make RR<1 favor treatment, initial resolution of symptoms.
|
|
viral shedding, 31.6% higher, RR 1.32, p = 0.24, treatment 59, control 57, inverted to make RR<1 favor treatment, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
13.
Cenikli et al., Does Favipiravir interact with DNA? Design of electrochemical DNA nanobiosensor to investigate the interaction between DNA and Favipiravir used in the treatment of COVID-19, Talanta, doi:10.1016/j.talanta.2025.128084.
Holubar et al., 24 Nov 2021, Double Blind Randomized Controlled Trial, USA, peer-reviewed, 26 authors, study period 8 July, 2020 - 23 March, 2021, average treatment delay 5.0 days, conflicts of interest:
Pfizer, Gates Foundation, Gilead, Regeneron, Janssen.
Favipiravir for treatment of outpatients with asymptomatic or uncomplicated COVID-19: a double-blind randomized, placebo-controlled, phase 2 trial
doi:10.1093/cid/ciac312/6572081
Background: Favipiravir is an oral, RNA-dependent RNA polymerase inhibitor with in vitro activity against SARS-CoV2. Despite limited data, favipiravir is administered to patients with COVID-19 in several countries.
Methods: We conducted a phase 2 double-blind randomized controlled outpatient trial of favipiravir in asymptomatic or mildly symptomatic adults with a positive SARS-CoV2 RT-PCR within 72 hours of enrollment. Participants were randomized 1:1 to receive placebo or favipiravir (1800 mg BID Day 1, 800mg BID Days 2-10). The primary outcome was SARS-CoV-2 shedding cessation in a modified intention-to-treat (mITT) cohort of participants with positive enrollment RT-PCRs. Using SARS-CoV-2 amplicon-based sequencing, we assessed favipiravir's impact on mutagenesis. Results: From July 8, 2020 -March 23, 2021, we randomized 149 participants with 116 included in the mITT cohort. The participants' mean age was 43 years (SD 12.5) and 57 (49%) were women. We found no difference in time to shedding cessation by treatment arm overall (HR 0.76 favoring placebo, 95% confidence interval [CI] 0.48 -1.20) or in sub-group analyses (age, sex, high-risk comorbidities, seropositivity or symptom duration at enrollment). We observed no difference in time to symptom resolution (initial: HR 0.84, 95% CI 0.54 -1.29; sustained: HR 0.87, 95% CI 0.52 -1.45). We detected no difference in accumulation of transition mutations in the viral genome during treatment. Conclusions: Our data do not support favipiravir use at commonly used doses in outpatients with uncomplicated COVID-19. Further research is needed to ascertain if higher doses of favipiravir are effective and safe for patients with COVID-19.
Participants We enrolled asymptomatic or symptomatic adults without respiratory distress who had a positive SARS-CoV-2 reverse-transcription polymerase chain reaction assay (RT-PCR) collected within 72 hours of enrollment. We excluded individuals who required renal replacement therapy, had liver impairment, were immunocompromised, or were pregnant or breast-feeding. See Supplementary Appendix for full criteria. Participants were randomized 1:1 to favipiravir or placebo using block, REDCap-implemented, randomization stratified by age (>=50 and <50 years old) and sex. [4, 5]
Procedures Participants received placebo or favipiravir 1800 mg BID on day 1, then 800mg BID on days 2-10. Favipiravir and placebo tablets were identical in appearance to maintain blinding. We followed participants for 28 days and performed a clinical assessment (including vital signs and targeted physical exams) and collected oropharyngeal (OP) swabs and blood samples at each visit. Staff-collected OP specimens underwent RT-PCR (Viroclinics Biosciences, Rotterdam, The Netherlands). Anti-SARSCoV-2 serology was performed using a virus plaque reduction neutralization assay (Viroclinics Biosciences, Rotterdam, The Netherlands). Participants self-collected daily anterior nasal swabs on days 1-10, 14, 21, and 28 and submitted them directly for RT-PCR with an assay that targeted the viral nucleocapsid gene's N1 and N3 regions (Quest Diagnostics, Secaucus, New Jersey). Participants also completed..
References
Abdelnabi, Foo, Kaptein, The combined treatment of Molnupiravir and Favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model, EBioMedicine
Andrews, The author reports research support from anonymous donors to Stanford University
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Cai, Yang, Liu, Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study, Engineering
Core, R: A language and environment for statistical computing
Doi, Hibino, Hase, A Prospective, Randomized, Open-Label Trial of Early versus Late Favipiravir Therapy in Hospitalized Patients with COVID-19, Antimicrob Agents Chemother
Ewels, Peltzer, Fillinger, The nf-core framework for community-curated bioinformatics pipelines, Nat Biotechnol
Fischer, Eron, Holman, an Oral Antiviral Treatment for COVID-19, medRxiv
Foundation, Academy of Pediatrics for the National Conference meeting, and is on a Pfizer data safety monitoring board (
Guedj, Piorkowski, Jacquot, Antiviral efficacy of favipiravir against Ebola virus: A translational study in cynomolgus macaques, PLoS Med
Harris, Taylor, Minor, The REDCap consortium: Building an international community of software platform partners, J Biomed Inform
Harris, Taylor, Thielke, Payne, Gonzalez et al., Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform
Ivashchenko, Dmitriev, Vostokova, AVIFAVIR for Treatment of Patients With Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of a Phase II/III Multicenter Randomized Clinical Trial, Clin Infect Dis
Jefferson, Spencer, Brassey, Heneghan, Viral cultures for COVID-19 infectious potential assessment -a systematic review, Clin Infect Dis
Kassambara, Rstatix, Pipe-Friendly Framework for Basic Statistical Tests
Khosla, Deans, Okesli, Morgens, Khosla et al., The author reports licenses from Clear Creak Bio and patents assigned to Stanford University, Patent #
Lee, Herigon, Benedetti, Pollock, Denkinger, Performance of Saliva, Oropharyngeal Swabs, and Nasal Swabs for SARS-CoV-2 Molecular Detection: a Systematic Review and Meta-analysis, J Clin Microbiol
Lou, Liu, Yao, Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: An Exploratory Randomized, Controlled Trial, Eur J Pharm Sci
Maldonado, The author reports grants from the NIH (U54, Pfizer
Shannon, Selisko, Le, Rapid incorporation of Favipiravir by the fast and permissive viral RNA polymerase complex results in SARS-CoV-2 lethal mutagenesis, Nat Commun
Sissoko, Laouenan, Folkesson, Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea, PLoS Med
Tomita, Takeda, Matsuyama, The anti-influenza virus drug favipiravir has little effect on replication of SARS-CoV-2 in cultured cells, Antimicrob Agents Chemother
Trotti, Colevas, Setser, CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment, Seminars in Radiation Oncology
Udwadia, Singh, Barkate, Efficacy and safety of favipiravir, an oral RNAdependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int J Infect Dis
Wang, Zhong, Salam, Phase 2a, open-label, dose-escalating, multi-center pharmacokinetic study of favipiravir (T-705) in combination with oseltamivir in patients with severe influenza, EBioMedicine
Zhou, Hill, Sarkar, -hydroxycytidine Inhibits SARS-CoV-2
DOI record:
{
"DOI": "10.1093/cid/ciac312",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciac312",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Favipiravir is an oral, RNA-dependent RNA polymerase inhibitor with in vitro activity against SARS-CoV2. Despite limited data, favipiravir is administered to patients with COVID-19 in several countries.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We conducted a phase 2 double-blind randomized controlled outpatient trial of favipiravir in asymptomatic or mildly symptomatic adults with a positive SARS-CoV2 RT-PCR within 72 hours of enrollment. Participants were randomized 1: 1 to receive placebo or favipiravir (1800mg BID Day 1, 800 mg BID Days 2-10). The primary outcome was SARS-CoV-2 shedding cessation in a modified intention-to-treat (mITT) cohort of participants with positive enrollment RT-PCRs. Using SARS-CoV-2 amplicon-based sequencing, we assessed favipiravir’s impact on mutagenesis.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>From July 8, 2020 - March 23, 2021, we randomized 149 participants with 116 included in the mITT cohort. The participants’ mean age was 43 years (SD 12.5) and 57 (49%) were women. We found no difference in time to shedding cessation by treatment arm overall (HR 0.76 favoring placebo, 95% confidence interval [CI] 0.48–1.20) or in sub-group analyses (age, sex, high-risk comorbidities, seropositivity or symptom duration at enrollment). We observed no difference in time to symptom resolution (initial: HR 0.84, 95% CI 0.54–1.29; sustained: HR 0.87, 95% CI 0.52–1.45). We detected no difference in accumulation of transition mutations in the viral genome during treatment.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Our data do not support favipiravir use at commonly used doses in outpatients with uncomplicated COVID-19. Further research is needed to ascertain if higher doses of favipiravir are effective and safe for patients with COVID-19.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7585-1809",
"affiliation": [
{
"name": "Division of Infectious Diseases and Geographic Medicine, Stanford University School of Medicine, Stanford, CA, USA"
}
],
"authenticated-orcid": false,
"family": "Holubar",
"given": "Marisa",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases and Geographic Medicine, Stanford University School of Medicine, Stanford, CA, USA"
}
],
"family": "Subramanian",
"given": "Aruna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Quantitative Sciences Unit, Division of Biomedical Informatics Research, Department of Medicine, Stanford University, Palo Alto, CA, USA"
}
],
"family": "Purington",
"given": "Natasha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Quantitative Sciences Unit, Division of Biomedical Informatics Research, Department of Medicine, Stanford University, Palo Alto, CA, USA"
}
],
"family": "Hedlin",
"given": "Haley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Quantitative Sciences Unit, Division of Biomedical Informatics Research, Department of Medicine, Stanford University, Palo Alto, CA, USA"
}
],
"family": "Bunning",
"given": "Bryan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases and Geographic Medicine, Stanford University School of Medicine, Stanford, CA, USA"
}
],
"family": "Walter",
"given": "Katharine S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases and Geographic Medicine, Stanford University School of Medicine, Stanford, CA, USA"
}
],
"family": "Bonilla",
"given": "Hector",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Stanford Center for Clinical Research, Stanford University, Stanford, CA, USA"
}
],
"family": "Boumis",
"given": "Athanasia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Stanford Solutions, Stanford University School of Medicine, Stanford, CA 94305, USA"
}
],
"family": "Chen",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Stanford Center for Clinical Research, Stanford University, Stanford, CA, USA"
}
],
"family": "Clinton",
"given": "Kimberly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Stanford Center for Clinical Research, Stanford University, Stanford, CA, USA"
}
],
"family": "Dewhurst",
"given": "Liisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Carol L. Epstein MD Consulting LLC, Wellington, FL, USA"
}
],
"family": "Epstein",
"given": "Carol",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6305-758X",
"affiliation": [
{
"name": "Division of Infectious Diseases and Geographic Medicine, Stanford University School of Medicine, Stanford, CA, USA"
},
{
"name": "Department of Microbiology and Immunology, Stanford University School of Medicine, Stanford, CA, USA"
}
],
"authenticated-orcid": false,
"family": "Jagannathan",
"given": "Prasanna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Stanford Solutions, Stanford University School of Medicine, Stanford, CA 94305, USA"
}
],
"family": "Kaszynski",
"given": "Richard H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Stanford Center for Clinical Research, Stanford University, Stanford, CA, USA"
}
],
"family": "Panu",
"given": "Lori",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases and Geographic Medicine, Stanford University School of Medicine, Stanford, CA, USA"
},
{
"name": "Department of Epidemiology and Population Health, Stanford University School of Medicine, Stanford, CA, USA"
}
],
"family": "Parsonnet",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Stanford ChEM-H, Stanford University, Stanford CA, USA"
}
],
"family": "Ponder",
"given": "Elizabeth L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases and Geographic Medicine, Stanford University School of Medicine, Stanford, CA, USA"
}
],
"family": "Quintero",
"given": "Orlando",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Stanford ChEM-H, Stanford University, Stanford CA, USA"
}
],
"family": "Sefton",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases and Geographic Medicine, Stanford University School of Medicine, Stanford, CA, USA"
},
{
"name": "Department of Microbiology and Immunology, Stanford University School of Medicine, Stanford, CA, USA"
}
],
"family": "Singh",
"given": "Upinder",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Stanford Center for Clinical Research, Stanford University, Stanford, CA, USA"
}
],
"family": "Soberanis",
"given": "Luke",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mariner Advanced Pharmacy Corp, San Mateo, California, USA"
}
],
"family": "Truong",
"given": "Henry",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases and Geographic Medicine, Stanford University School of Medicine, Stanford, CA, USA"
}
],
"family": "Andrews",
"given": "Jason R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Quantitative Sciences Unit, Division of Biomedical Informatics Research, Department of Medicine, Stanford University, Palo Alto, CA, USA"
}
],
"family": "Desai",
"given": "Manisha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Departments of Chemistry and Chemical Engineering, Stanford University, Stanford, CA, USA"
},
{
"name": "Stanford ChEM-H, Stanford University, Stanford CA, USA"
}
],
"family": "Khosla",
"given": "Chaitan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pediatrics, Stanford University School of Medicine, Stanford, CA, USA"
},
{
"name": "Department of Epidemiology and Population Health, Stanford University School of Medicine, Stanford, CA, USA"
}
],
"family": "Maldonado",
"given": "Yvonne",
"sequence": "additional"
}
],
"container-title": [
"Clinical Infectious Diseases"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
4,
20
]
],
"date-time": "2022-04-20T19:16:25Z",
"timestamp": 1650482185000
},
"deposited": {
"date-parts": [
[
2022,
4,
21
]
],
"date-time": "2022-04-21T18:22:18Z",
"timestamp": 1650565338000
},
"indexed": {
"date-parts": [
[
2022,
4,
21
]
],
"date-time": "2022-04-21T21:44:35Z",
"timestamp": 1650577475037
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "1058-4838"
},
{
"type": "electronic",
"value": "1537-6591"
}
],
"issued": {
"date-parts": [
[
2022,
4,
21
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
21
]
],
"date-time": "2022-04-21T00:00:00Z",
"timestamp": 1650499200000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac312/43407130/ciac312.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac312/43407130/ciac312.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
4,
21
]
]
},
"published-online": {
"date-parts": [
[
2022,
4,
21
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciac312/6572081"
}
},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": [
"Favipiravir for treatment of outpatients with asymptomatic or uncomplicated COVID-19: a double-blind randomized, placebo-controlled, phase 2 trial"
],
"type": "journal-article"
}