The DAWN antivirals trial: process evaluation of a COVID-19 trial in general practice
et al., BJGP Open, doi:10.3399/bjgpo.2023.0109, DAWN, NCT04730206, Nov 2023
Very small early terminated RCT with 8 molnupiravir and 17 placebo patients showing worse recovery with molnupiravir, without statistical significance.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Study covers TMPRSS2 inhibitors, camostat, and molnupiravir.
|
risk of no recovery, 82.9% higher, RR 1.83, p = 0.36, treatment 4 of 7 (57.1%), control 5 of 16 (31.2%), day 30.
|
|
risk of no recovery, no change, RR 1.00, p = 1.00, treatment 6 of 8 (75.0%), control 12 of 16 (75.0%), day 8.
|
|
recovery time, 135.0% higher, relative time 2.35, treatment 8, control 17.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Tare et al., 20 Nov 2023, Randomized Controlled Trial, placebo-controlled, Belgium, peer-reviewed, median age 55.0, 11 authors, study period June 2021 - July 2022, trial NCT04730206 (history) (DAWN).
Contact: ann.vandenbruel@kuleuven.be.
The DAWN antivirals trial: process evaluation of a COVID-19 trial in general practice
BJGP Open, doi:10.3399/bjgpo.2023.0109
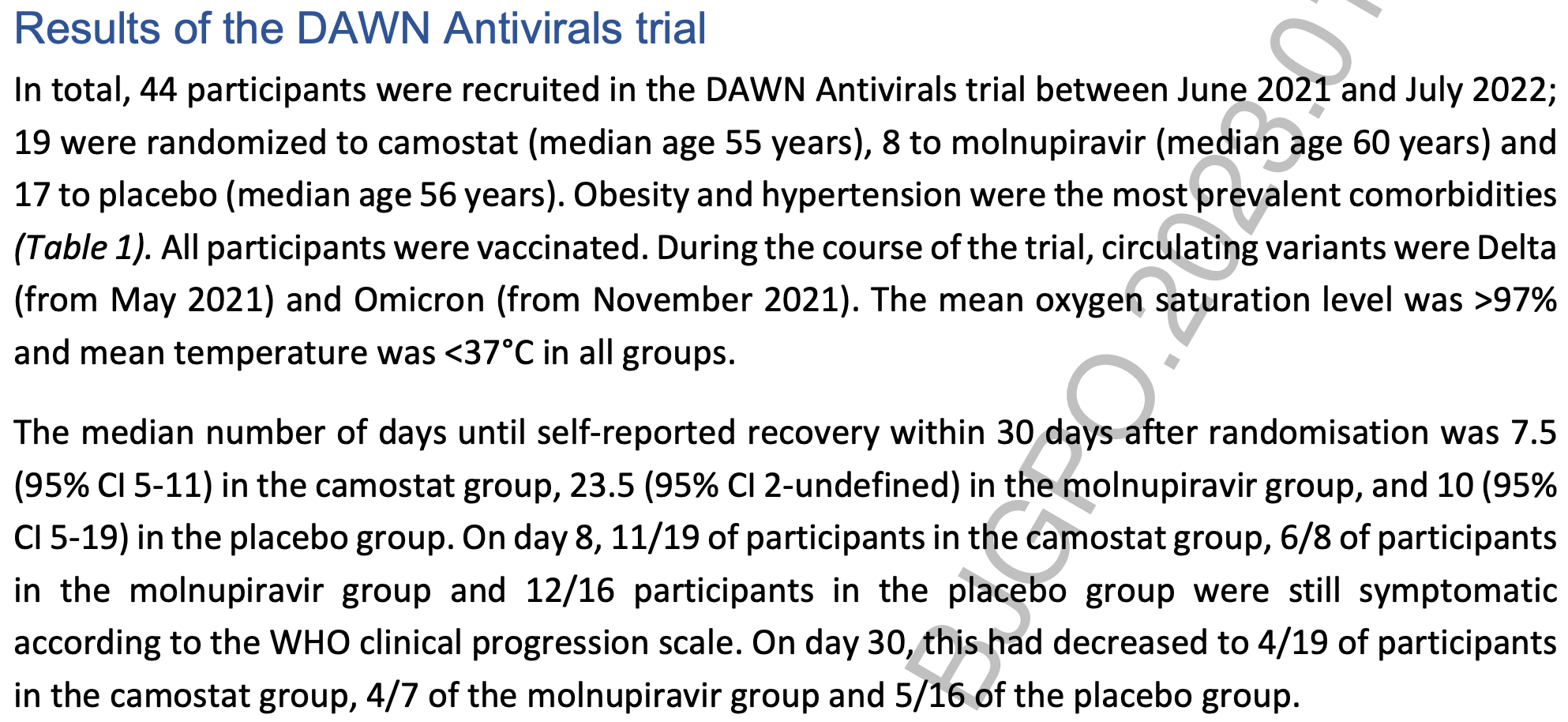
Background: The DAWN Antivirals trial was a multicentric, randomised placebo-controlled trial evaluating antiviral medication for COVID-19 in general practice. The trial was prematurely terminated because of insufficient recruitment. Aim: To explore which factors contributed to the premature termination. Design and setting: General practice in Belgium. Methods: Patients were randomized to camostat or placebo (blinded) between June 2021 and July 2022); a third arm evaluating molnupiravir (open label) was opened in March 2022. We analysed available trial data and evaluated trial context, implementation and mechanisms of impact based on semi-structured interviews with trial stakeholders. Results: The trial recruited 44 participants; 19 were allocated to camostat (median age 55 years), 8 to molnupiravir (median age 60 years) and 17 to placebo (median age 56 years). There were no serious adverse events in either group. Most difficulties were related to the pandemic context: disruption to routine clinical services; multiple changes to the service model for COVID-19 patients; overwhelmed clinical staff; delays of trial medication; staff shortages in the sponsor and clinical team. In addition, regulatory approval processes were lengthy and led to additional study procedures. It was felt that the trial started too late, when vaccinations had already begun.
Conclusion: The DAWN Antivirals trial was stopped prematurely. Although many barriers were related to the pandemic itself, hurdles such as small and inexperienced sponsor and clinical teams, delays in regulatory processes and research capacity in routine settings could be overcome by established research infrastructure and standardization of processes.
Author Accepted Manuscript This is an 'author accepted manuscript': a manuscript that has been accepted for publication in BJGP Open, but which has not yet undergone subediting, typesetting, or correction. Errors discovered and corrected during this process may materially alter the content of this manuscript, and the latest published version (the Version of Record) should be used in preference to any preceding versions
Discussion
Summary The DAWN Antivirals trial was set up when there was still an urgent need for effective treatment of COVID-19 patients in general practice. Recruitment was however insufficient and the trial was subsequently halted after 13 months. There were 12 months between trial conception and the actual start, largely due to the lengthy pre-submission advice process, delays of trial medication, and difficulties in finding staff. As a result, the trial opened when vaccination campaigns were well advanced, leading to a lower risk of serious illness and a subsequent change in endpoint, but also to decreased interest in a trial for a disease that was no longer considered a major threat. The pandemic itself had a huge impact on the trial: recruitment settings changed from testing centres to general practices to a decentralised system with remote informed consent; general practice was overwhelmed; study nurses were in short supply and charging very high rates; the number of potential participants was highly dependent on virus circulation in the population...
References
Angus, Berry, Lewis, The REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-acquired Pneumonia) Study. Rationale and Design, Ann Am Thorac Soc, doi:10.1513/AnnalsATS.202003-192SD
Bongard, Van Der Velden, Cook, Antivirals for influenza-Like Illness? A randomised Controlled trial of Clinical and Cost effectiveness in primary CarE (ALIC(4) E): the ALIC(4) E protocol, BMJ Open, doi:10.1136/bmjopen-2017-021032
Butler, Hobbs, Gbinigie, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet, doi:10.1016/S0140-6736(22)02597-1
Butler, Van Der Velden, Bongard, Oseltamivir plus usual care versus usual care for influenza-like illness in primary care: an open-label, pragmatic, randomised controlled trial, Lancet, doi:10.1016/S0140-6736(19)32982-4
Goossens, Derde, Horby, The European clinical research response to optimise treatment of patients with COVID-19: lessons learned, future perspective, and recommendations, Lancet Infect Dis, doi:10.1016/S1473-3099(21)00705-2
Grenier, Loniewski, Plazy, Implementing an outpatient clinical trial on COVID-19 treatment in an emergency epidemic context: a mixed methods study among operational and research stakeholders within the Coverage trial, Bordeaux (France), Arch Public Health, doi:10.1186/s13690-022-00999-9
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Ingelbeen, Peckeu, Laga, Reducing contacts to stop SARS-CoV-2 transmission during the second pandemic wave in Brussels, Belgium, August to, Euro Surveill, doi:10.2807/1560-7917.ES.2021.26.7.2100065
Kitagawa, Arai, Iida, A phase I study of high dose camostat mesylate in healthy adults provides a rationale to repurpose the TMPRSS2 inhibitor for the treatment of COVID-19, Clin Transl Sci, doi:10.1111/cts.13052
Mccarthy, Naggie, Boulware, Fluvoxamine for Outpatient Treatment of COVID-19: A Decentralized, Placebo-controlled, Randomized, Platform Clinical Trial, doi:10.1101/2022.10.17.22281178
Painter, Holman, Bush, Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity Against SARS-CoV-2, Antimicrob Agents Chemother, doi:10.1128/AAC.02428-20
Reis, Santos Moreira-Silva, Silva, Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial, Lancet Glob Health, doi:10.1016/S2214-109X(21)00448-4
Sciensano, COVID-19 Epidemiologisch Bulletin
Vandael, Latour, Islamaj, COVID-19 cases, hospitalizations and deaths in Belgian nursing homes: results of a surveillance conducted between April and December 2020, Arch Public Health, doi:10.1186/s13690-022-00794-6
Yu, Bafadhel, Dorward, Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet, doi:10.1016/S0140-6736(21)01744-X
DOI record:
{
"DOI": "10.3399/bjgpo.2023.0109",
"ISSN": [
"2398-3795"
],
"URL": "http://dx.doi.org/10.3399/bjgpo.2023.0109",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>The DAWN Antivirals trial was a multicentric, randomised placebo-controlled trial evaluating antiviral medication for COVID-19 in general practice. The trial was prematurely terminated because of insufficient recruitment.</jats:p></jats:sec><jats:sec><jats:title>Aim</jats:title><jats:p>To explore which factors contributed to the premature termination.</jats:p></jats:sec><jats:sec><jats:title>Design & setting</jats:title><jats:p>General practice in Belgium.</jats:p></jats:sec><jats:sec><jats:title>Method</jats:title><jats:p>Patients were randomized to camostat or placebo (blinded) between June 2021 and July 2022); a third arm evaluating molnupiravir (open label) was opened in March 2022. We analysed available trial data and evaluated trial context, implementation and mechanisms of impact based on semi-structured interviews with trial stakeholders.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>The trial recruited 44 participants; 19 were allocated to camostat (median age 55 years), 8 to molnupiravir (median age 60 years) and 17 to placebo (median age 56 years). There were no serious adverse events in either group. Most difficulties were related to the pandemic context: disruption to routine clinical services; multiple changes to the service model for COVID-19 patients; overwhelmed clinical staff; delays of trial medication; staff shortages in the sponsor and clinical team. In addition, regulatory approval processes were lengthy and led to additional study procedures. It was felt that the trial started too late, when vaccinations had already begun.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>The DAWN Antivirals trial was stopped prematurely. Although many barriers were related to the pandemic itself, hurdles such as small and inexperienced sponsor and clinical teams, delays in regulatory processes and research capacity in routine settings could be overcome by established research infrastructure and standardization of processes.</jats:p></jats:sec>",
"alternative-id": [
"10.3399/BJGPO.2023.0109"
],
"author": [
{
"affiliation": [],
"family": "Tare",
"given": "Dajana",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1238-8052",
"affiliation": [],
"authenticated-orcid": false,
"family": "Coenen",
"given": "Samuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Sutter",
"given": "An",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heytens",
"given": "Stefan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Devroey",
"given": "Dirk",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buret",
"given": "Laetitia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1909-9613",
"affiliation": [],
"authenticated-orcid": false,
"family": "Schoenmakers",
"given": "Birgitte",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Delvaux",
"given": "Nicolas",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7166-7211",
"affiliation": [],
"authenticated-orcid": false,
"family": "Verbakel",
"given": "Jan Y",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bogaerts",
"given": "Kris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van den Bruel",
"given": "Ann",
"sequence": "additional"
}
],
"container-title": "BJGP Open",
"container-title-short": "BJGP Open",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
11,
20
]
],
"date-time": "2023-11-20T13:40:11Z",
"timestamp": 1700487611000
},
"deposited": {
"date-parts": [
[
2023,
11,
20
]
],
"date-time": "2023-11-20T13:40:11Z",
"timestamp": 1700487611000
},
"indexed": {
"date-parts": [
[
2023,
11,
21
]
],
"date-time": "2023-11-21T00:14:19Z",
"timestamp": 1700525659799
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
11,
20
]
]
},
"language": "en",
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.3399/BJGPO.2023.0109",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1987",
"original-title": [],
"page": "BJGPO.2023.0109",
"prefix": "10.3399",
"published": {
"date-parts": [
[
2023,
11,
20
]
]
},
"published-online": {
"date-parts": [
[
2023,
11,
20
]
]
},
"publisher": "Royal College of General Practitioners",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://bjgpopen.org/lookup/doi/10.3399/BJGPO.2023.0109"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Family Practice"
],
"subtitle": [],
"title": "The DAWN antivirals trial: process evaluation of a COVID-19 trial in general practice",
"type": "journal-article"
}