Hydroxyzine Use and Mortality in Patients Hospitalized for COVID-19: A Multicenter Observational Study
et al., Journal of Clinical Medicine, doi:10.3390/jcm10245891, Dec 2021
11th treatment shown to reduce risk in
December 2020, now with p = 0.000052 from 17 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
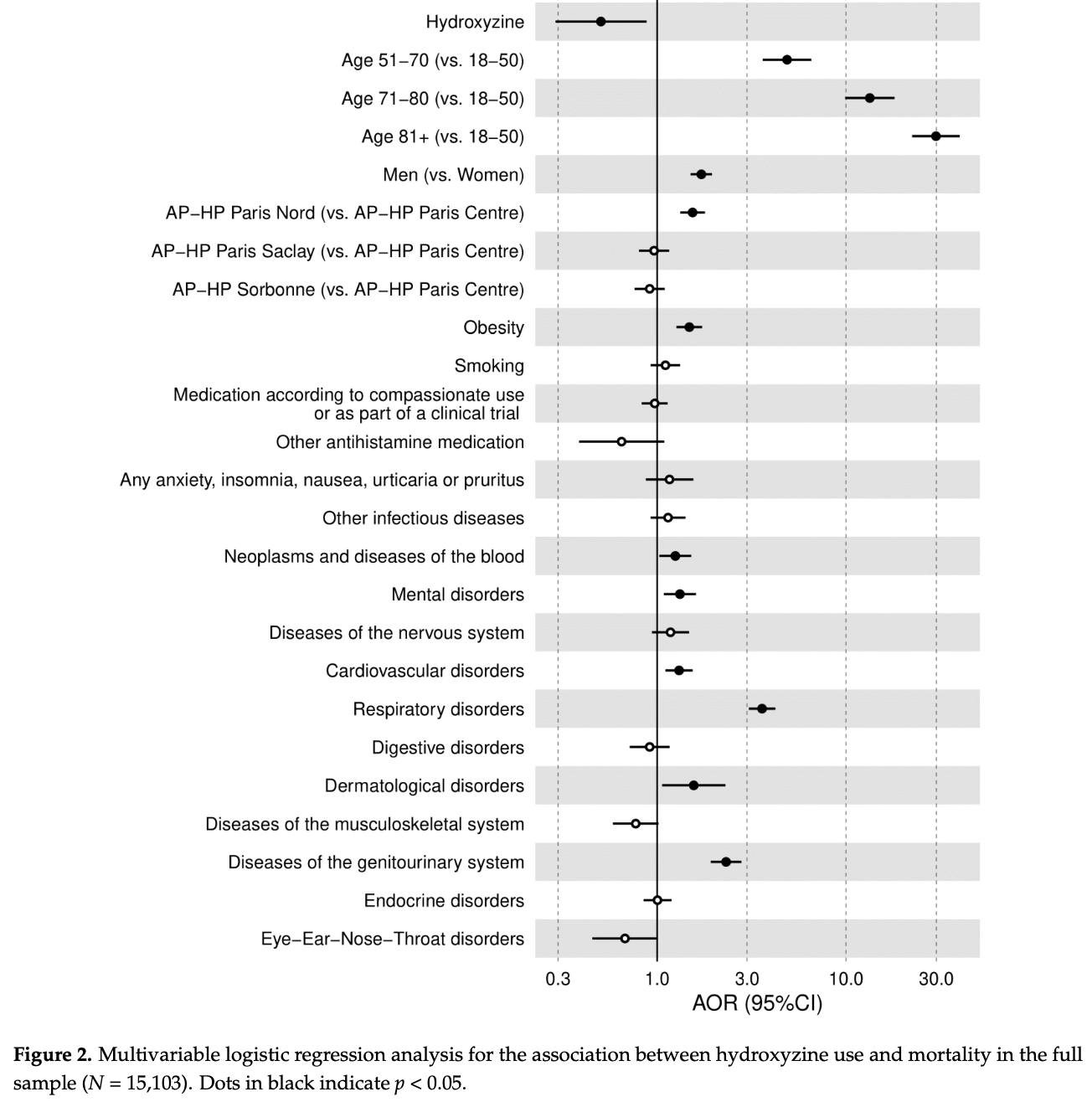
Retrospective 15,103 hospitalized COVID-19 patients in France showing lower mortality with hydroxyzine use.
|
risk of death, 46.2% lower, RR 0.54, p = 0.02, treatment 18 of 164 (11.0%), control 1,571 of 14,939 (10.5%), adjusted per study, odds ratio converted to relative risk, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sánchez-Rico et al., 15 Dec 2021, retrospective, France, peer-reviewed, 20 authors, study period 24 January, 2020 - 1 May, 2020.
Contact: marinals@ucm.es (corresponding author), frederic.limosin@aphp.fr, cedric.lemogne@aphp.fr, pedelamu@ucm.com, nico.hoertel@yahoo.fr, vernet.raphael@gmail.com, nathanael.beeker@aphp.fr, antoine.neuraz@aphp.fr, anita.burgun@aphp.fr, carlos.blanco2@nih.gov, mark.olfson@nyspi.columbia.edu, pierre.meneton@spim.jussieu.fr, christel.daniel@aphp.fr, nicolas.paris@aphp.fr, alexandre.gramfort@inria.fr, guillaume.lemaitre@inria.fr, elisa.salamanca@aphp.fr, melodie.bernaux@aphp.fr, ali.bellamine@aphp.fr.
Hydroxyzine Use and Mortality in Patients Hospitalized for COVID-19: A Multicenter Observational Study
Journal of Clinical Medicine, doi:10.3390/jcm10245891
1) Background: Based on its antiviral activity, anti-inflammatory properties, and functional inhibition effects on the acid sphingomyelinase/ceramide system (FIASMA), we sought to examine the potential usefulness of the H1 antihistamine hydroxyzine in patients hospitalized for COVID-19. (2) Methods: In a multicenter observational study, we included 15,103 adults hospitalized for COVID-19, of which 164 (1.1%) received hydroxyzine within the first 48 h of hospitalization, administered orally at a median daily dose of 25.0 mg (SD = 29.5). We compared mortality rates between patients who received hydroxyzine at hospital admission and those who did not, using a multivariable logistic regression model adjusting for patients' characteristics, medical conditions, and use of other
Acknowledgments: The authors thank the EDS APHP COVID consortium integrating the APHP Health Data Warehouse team as well as all the APHP staff and volunteers who contributed to the implementation of the EDS-COVID database and operating solutions for this database. Collaborators of the EDS APHP COVID consortium are: Pierre-Yves Ancel, Alain Bauchet, Nathanaël Beeker, Vincent Benoit, Mélodie Bernaux, Ali Bellamine, Romain Bey, Aurélie Bourmaud, Stéphane Breant, Anita Burgun, Fabrice Carrat, Charlotte Caucheteux, Julien Champ, Sylvie Cormont, Christel Daniel, Julien Dubiel, Catherine Ducloas, Loic Esteve, Marie Frank, Nicolas Garcelon, Alexandre Gramfort, Nicolas Griffon, Olivier Grisel, Martin Guilbaud, Claire Hassen-Khodja, François Hemery, Martin Hilka, Anne-Sophie Jannot, Jerome Lambert, Richard Layese, Judith Leblanc, Léo Lebouter, Guillaume Lemaitre, Damien Leprovost, Ivan Lerner, Kankoe Levi Sallah, Aurélien Maire, Marie-France Mamzer, Patricia Martel, Arthur Mensch, Thomas Moreau, Antoine Neuraz, Nina Orlova, Nicolas Paris, Bastien Rance, Hélène Ravera, Antoine Rozes, Elisa Salamanca, Arnaud Sandrin, Patricia Serre, Xavier Tannier, Jean-Marc Treluyer, Damien Van Gysel, Gaël Varoquaux, Jill Jen Vie, Maxime Wack, Perceval Wajsburt, Demian Wassermann, Eric Zapletal.
Conflicts of Interest: All authors have completed the Unified Competing Interest form (available on request from the corresponding author) and declare: no support from any organization for the submitted work;..
References
Austin, Using the Standardized Difference to Compare the Prevalence of a Binary Variable between Two Groups in Observational Research, Commun. Stat.-Simul. Comput, doi:10.1080/03610910902859574
Blaess, Kaiser, Sommerfeld, Rentschler, Csuk et al., Rational Drug Repurposing: Focus on Lysosomotropism, Targets in Disease Process, Drug Profile, and Pulmonary Tissue Accumulation in SARS-CoV-2 Infection/COVID-19, Front. Pharmacol, doi:10.3389/fphar.2020.584881
Carpinteiro, Edwards, Hoffmann, Kochs, Gripp et al., Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells, Cell Rep. Med, doi:10.1016/j.xcrm.2020.100142
Carpinteiro, Gripp, Hoffmann, Pöhlmann, Hoertel et al., Inhibition of Acid Sphingomyelinase by Ambroxol Prevents SARS-CoV-2 Entry into Epithelial Cells, J. Biol. Chem, doi:10.1016/j.jbc.2021.100701
Chevance, Gourion, Hoertel, Llorca, Thomas et al., Ensuring mental health care during the SARS-CoV-2 epidemic in France: A narrative review, L'Encephale, doi:10.1016/j.encep.2020.03.001
Darquennes, Le Corre, Le Moine, Loas, Association between Functional Inhibitors of Acid Sphingomyelinase (Fiasmas) and Reduced Risk of Death in COVID-19 Patients: A Retrospective Cohort Study, Pharmaceuticals, doi:10.3390/ph14030226
Dei Cas, Ottolenghi, Morano, Rinaldo, Roda et al., Link between Serum Lipid Signature and Prognostic Factors in COVID-19 Patients, Sci. Rep, doi:10.1038/s41598-021-00755-z
Elm, Altman, Egger, Pocock, Gøtzsche et al., The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies, Ann. Intern. Med, doi:10.7326/0003-4819-147-8-200710160-00010
Ge, Wang, Hou, Lv, Wang et al., Repositioning of Histamine H1 Receptor Antagonist: Doxepin Inhibits Viropexis of SARS-CoV-2 Spike Pseudovirus by Blocking ACE2, Eur. J. Pharmacol, doi:10.1016/j.ejphar.2021.173897
Gordon, Jang, Bouhaddou, Xu, Obernier et al., A SARS-CoV-2 Protein Interaction Map Reveals Targets for Drug Repurposing, Nature, doi:10.1038/s41586-020-2286-9
Hoertel, Blachier, Blanco, Olfson, Massetti et al., A Stochastic Agent-Based Model of the SARS-CoV-2 Epidemic in France, Nat. Med, doi:10.1038/s41591-020-1001-6
Hoertel, Blachier, Blanco, Olfson, Massetti et al., Facing the COVID-19 Epidemic in NYC: A Stochastic Agent-Based Model of Various Intervention Strategies, medRxiv, doi:10.1101/2020.04.23.20076885
Hoertel, Blachier, Sánchez-Rico, Limosin, Leleu, Impact of the Timing and Adherence to Face Mask Use on the Course of the COVID-19 Epidemic in France, J. Travel Med, doi:10.1093/jtm/taab016
Hoertel, Do the Selective Serotonin Reuptake Inhibitor Antidepressants Fluoxetine and Fluvoxamine Reduce Mortality among Patients with COVID-19?, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2021.36510
Hoertel, Sánchez-Rico, Cougoule, Gulbins, Kornhuber et al., Repurposing Antidepressants Inhibiting the Sphingomyelinase Acid/Ceramide System against COVID-19: Current Evidence and Potential Mechanisms, Mol. Psychiatry, doi:10.1038/s41380-021-01254-3
Hoertel, Sánchez-Rico, Gulbins, Kornhuber, Carpinteiro et al., Association Between FIASMAs and Reduced Risk of Intubation or Death in Individuals Hospitalized for Severe COVID-19: An Observational Multicenter Study, Clin. Pharmacol. Ther, doi:10.1002/cpt.2317
Hoertel, Sánchez-Rico, Gulbins, Kornhuber, Carpinteiro et al., Association between Psychotropic Medications Functionally Inhibiting Acid Sphingomyelinase and Reduced Risk of Intubation or Death among Individuals with Mental Disorder and Severe COVID-19: An Observational Study, medRxiv, doi:10.1101/2021.02.18.21251997
Hoertel, Sánchez-Rico, Vernet, Beeker, Jannot et al., Association between Antidepressant Use and Reduced Risk of Intubation or Death in Hospitalized Patients with COVID-19: Results from an Observational Study, Mol. Psychiatry, doi:10.1038/s41380-021-01021-4
Hoertel, Sánchez-Rico, Vernet, Beeker, Neuraz et al., Dexamethasone Use and Mortality in Hospitalized Patients with Coronavirus Disease 2019: A Multicentre Retrospective Observational Study, Br. J. Clin. Pharmacol, doi:10.1111/bcp.14784
Hoertel, Sánchez-Rico, Vernet, Jannot, Neuraz et al., Observational Study of Chlorpromazine in Hospitalized Patients with COVID-19, Clin. Drug Investig, doi:10.1007/s40261-021-01001-0
Hoertel, Sánchez-Rico, Vernet, Jannot, Neuraz et al., Observational Study of Haloperidol in Hospitalized Patients with COVID-19, PLoS ONE, doi:10.1371/journal.pone.0247122
Kornhuber, Hoertel, Gulbins, The Acid Sphingomyelinase/Ceramide System in COVID-19, Mol. Psychiatry, doi:10.1038/s41380-021-01309-5
Kornhuber, Muehlbacher, Trapp, Pechmann, Friedl et al., Identification of Novel Functional Inhibitors of Acid Sphingomyelinase, PLoS ONE, doi:10.1371/journal.pone.0023852
Kornhuber, Tripal, Reichel, Mühle, Rhein et al., Functional Inhibitors of Acid Sphingomyelinase (FIASMAs): A Novel Pharmacological Group of Drugs with Broad Clinical Applications, Cell. Physiol. Biochem, doi:10.1159/000315101
Lagunas-Rangel, Neutrophil-to-Lymphocyte Ratio and Lymphocyte-to-C-Reactive Protein Ratio in Patients with Severe Coronavirus Disease 2019 (COVID-19): A Meta-Analysis, J. Med. Virol, doi:10.1002/jmv.25819
Lenze, Mattar, Zorumski, Stevens, Schweiger et al., Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients with Symptomatic COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.22760
Liu, Yan, Wan, Xiang, Le et al., Viral Dynamics in Mild and Severe Cases of COVID-19, Lancet Infect. Dis, doi:10.1016/S1473-3099(20)30232-2
Liu, Zhang, Joo, Sun, Nf-Kb, Signaling in Inflammation, Signal Transduct. Target. Ther, doi:10.1038/sigtrans.2017.23
Mandhane, Shah, Bahekar, Mehetre, Pawar et al., Characterization of Anti-Inflammatory Properties and Evidence for No Sedation Liability for the Novel Antihistamine SUN-1334, H. Int. Arch. Allergy Immunol, doi:10.1159/000232571
Marín-Corral, Rodríguez-Morató, Gomez-Gomez, Pascual-Guardia, Muñoz-Bermúdez et al., Metabolic Signatures Associated with Severity in Hospitalized COVID-19 Patients, Int. J. Mol. Sci, doi:10.3390/ijms22094794
Matta, Wiernik, Robineau, Carrat, Touvier et al., Association of Self-Reported COVID-19 Infection and SARS-CoV-2 Serology Test Results with Persistent Physical Symptoms among French Adults during the COVID-19 Pandemic, JAMA Intern. Med, doi:10.1001/jamainternmed.2021.6454
Norinder, Tuck, Norgren, Munic Kos, Existing Highly Accumulating Lysosomotropic Drugs with Potential for Repurposing to Target COVID-19, Biomed. Pharmacother. Biomed. Pharmacother, doi:10.1016/j.biopha.2020.110582
Reis, Dos, Moreira-Silva, Silva, Thabane et al., Effect of Early Treatment with Fluvoxamine on Risk of Emergency Care and Hospitalisation among Patients with COVID-19: The TOGETHER Randomised, Platform Clinical Trial, Lancet Glob. Health, doi:10.1016/S2214-109X(21)00448-4
Reznikov, Norris, Vashisht, Bluhm, Li et al., Identification of Antiviral Antihistamines for COVID-19 Repurposing, Biochem. Biophys. Res. Commun, doi:10.1016/j.bbrc.2020.11.095
Rivas, Saponi-Cortes, Zamorano, Hydroxyzine Inhibits SARS-CoV-2 Spike Protein Binding to ACE2 in a Qualitative in Vitro Assay, bioRxiv, doi:10.1101/2021.01.04.424792
Rosen, Seki, Fernández-Castañeda, Beiter, Eccles et al., Modulation of the Sigma-1 Receptor-IRE1 Pathway Is Beneficial in Preclinical Models of Inflammation and Sepsis, Sci. Transl. Med, doi:10.1126/scitranslmed.aau5266
Rosenbaum, Rubin, The Central Role of the Propensity Score in Observational Studies for Causal Effects, Biometrika, doi:10.1093/biomet/70.1.41
Rosenwasser, New Insights into the Pathophysiology of Allergic Rhinitis, Allergy Asthma Proc, doi:10.2500/aap.2007.28.2977
Schloer, Brunotte, Goretzko, Mecate-Zambrano, Korthals et al., Targeting the Endolysosomal Host-SARS-CoV-2 Interface by Clinically Licensed Functional Inhibitors of Acid Sphingomyelinase (FIASMA) including the Antidepressant Fluoxetine, Emerg. Microbes Infect, doi:10.1080/22221751.2020.1829082
Schneeweiss, Sensitivity Analysis and External Adjustment for Unmeasured Confounders in Epidemiologic Database Studies of Therapeutics, Pharmacoepidemiol. Drug Saf, doi:10.1002/pds.1200
Seftel, Boulware, Prospective Cohort of Fluvoxamine for Early Treatment of Coronavirus Disease 19, Open Forum Infect. Dis, doi:10.1093/ofid/ofab050
Serdar, Cihan, Yücel, Serdar, Sample Size, Power and Effect Size Revisited: Simplified and Practical Approaches in Pre-Clinical, Clinical and Laboratory Studies, Biochem. Med, doi:10.11613/BM.2021.010502
Stebbing, Phelan, Griffin, Tucker, Oechsle et al., COVID-19: Combining Antiviral and Anti-Inflammatory Treatments, Lancet Infect. Dis, doi:10.1016/S1473-3099(20)30132-8
Sukhatme, Reiersen, Vayttaden, Sukhatme, Fluvoxamine: A Review of Its Mechanism of Action and Its Role in COVID-19, Front. Pharmacol, doi:10.3389/fphar.2021.652688
Torretta, Poliseno, Capitanio, Biasin, Santantonio et al., Severity of COVID-19 Patients Predicted by Serum Sphingolipids Signature, Int. J. Mol. Sci, doi:10.3390/ijms221910198
Vela, Repurposing Sigma-1 Receptor Ligands for COVID-19 Therapy?, Front. Pharmacol, doi:10.3389/fphar.2020.582310
Villoutreix, Beaune, Tamouza, Krishnamoorthy, Leboyer, Prevention of COVID-19 by Drug Repurposing: Rationale from Drugs Prescribed for Mental Disorders, Drug Discov. Today, doi:10.1016/j.drudis.2020.06.022
Villoutreix, Krishnamoorthy, Tamouza, Leboyer, Beaune, Chemoinformatic Analysis of Psychotropic and Antihistaminic Drugs in the Light of Experimental Anti-SARS-CoV-2 Activities, Adv. Appl. Bioinforma. Chem, doi:10.2147/AABC.S304649
Williamson, Walker, Bhaskaran, Bacon, Bates et al., Factors Associated with COVID-19-Related Death Using OpenSAFELY, Nature, doi:10.1038/s41586-020-2521-4
Zhou, Dai, Tong, COVID-19: A Recommendation to Examine the Effect of Hydroxychloroquine in Preventing Infection and Progression, J. Antimicrob. Chemother, doi:10.1093/jac/dkaa114
DOI record:
{
"DOI": "10.3390/jcm10245891",
"ISSN": [
"2077-0383"
],
"URL": "http://dx.doi.org/10.3390/jcm10245891",
"abstract": "<jats:p>(1) Background: Based on its antiviral activity, anti-inflammatory properties, and functional inhibition effects on the acid sphingomyelinase/ceramide system (FIASMA), we sought to examine the potential usefulness of the H1 antihistamine hydroxyzine in patients hospitalized for COVID-19. (2) Methods: In a multicenter observational study, we included 15,103 adults hospitalized for COVID-19, of which 164 (1.1%) received hydroxyzine within the first 48 h of hospitalization, administered orally at a median daily dose of 25.0 mg (SD = 29.5). We compared mortality rates between patients who received hydroxyzine at hospital admission and those who did not, using a multivariable logistic regression model adjusting for patients’ characteristics, medical conditions, and use of other medications. (3) Results: This analysis showed a significant association between hydroxyzine use and reduced mortality (AOR, 0.51; 95%CI, 0.29–0.88, p = 0.016). This association was similar in multiple sensitivity analyses. (4) Conclusions: In this retrospective observational multicenter study, the use of the FIASMA hydroxyzine was associated with reduced mortality in patients hospitalized for COVID-19. Double-blind placebo-controlled randomized clinical trials of hydroxyzine for COVID-19 are needed to confirm these results, as are studies to examine the potential usefulness of this medication for outpatients and as post-exposure prophylaxis for individuals at high risk for severe COVID-19.</jats:p>",
"alternative-id": [
"jcm10245891"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-1121-8641",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sánchez-Rico",
"given": "Marina",
"sequence": "first"
},
{
"affiliation": [],
"family": "Limosin",
"given": "Frédéric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vernet",
"given": "Raphaël",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beeker",
"given": "Nathanaël",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Neuraz",
"given": "Antoine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Blanco",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Olfson",
"given": "Mark",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3487-4721",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lemogne",
"given": "Cédric",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4611-1892",
"affiliation": [],
"authenticated-orcid": false,
"family": "Meneton",
"given": "Pierre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daniel",
"given": "Christel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paris",
"given": "Nicolas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gramfort",
"given": "Alexandre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lemaitre",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De La Muela",
"given": "Pedro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salamanca",
"given": "Elisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bernaux",
"given": "Mélodie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bellamine",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Burgun",
"given": "Anita",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7890-1349",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hoertel",
"given": "Nicolas",
"sequence": "additional"
},
{
"affiliation": [],
"name": "on behalf of AP-HP/Université de Paris/INSERM COVID-19 Research Collaboration/AP-HP COVID CDR Initiative/“Entrepôt de Données de Santé” AP-HP Consortium",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical Medicine",
"container-title-short": "JCM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
15
]
],
"date-time": "2021-12-15T14:02:19Z",
"timestamp": 1639576939000
},
"deposited": {
"date-parts": [
[
2021,
12,
15
]
],
"date-time": "2021-12-15T15:00:34Z",
"timestamp": 1639580434000
},
"indexed": {
"date-parts": [
[
2024,
4,
22
]
],
"date-time": "2024-04-22T14:28:13Z",
"timestamp": 1713796093365
},
"is-referenced-by-count": 9,
"issue": "24",
"issued": {
"date-parts": [
[
2021,
12,
15
]
]
},
"journal-issue": {
"issue": "24",
"published-online": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
15
]
],
"date-time": "2021-12-15T00:00:00Z",
"timestamp": 1639526400000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2077-0383/10/24/5891/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "5891",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
12,
15
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
15
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1038/s41591-020-1001-6",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1093/jtm/taab016",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1101/2020.04.23.20076885",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1001/jamainternmed.2021.6454",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1016/j.encep.2020.03.001",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1001/jamanetworkopen.2021.36510",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1038/s41380-021-01254-3",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.2500/aap.2007.28.2977",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1159/000232571",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1038/sigtrans.2017.23",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1016/j.drudis.2020.06.022",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.2147/AABC.S304649",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1101/2021.01.04.424792",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1016/j.bbrc.2020.11.095",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1016/j.ejphar.2021.173897",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1038/s41380-021-01309-5",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1371/journal.pone.0023852",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1159/000315101",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1016/j.xcrm.2020.100142",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1016/j.jbc.2021.100701",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1080/22221751.2020.1829082",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1002/cpt.2317",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.3390/ph14030226",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1093/ofid/ofab050",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1001/jama.2020.22760",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1016/S2214-109X(21)00448-4",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1101/2021.02.18.21251997",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.3390/ijms22094794",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1038/s41598-021-00755-z",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.3390/ijms221910198",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.3389/fphar.2020.582310",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1126/scitranslmed.aau5266",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.3389/fphar.2021.652688",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1093/biomet/70.1.41",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1016/j.biopha.2020.110582",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.3389/fphar.2020.584881",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1093/jac/dkaa114",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1016/S1473-3099(20)30132-8",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1002/jmv.25819",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1016/S1473-3099(20)30232-2",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1038/s41380-021-01021-4",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1111/bcp.14784",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1007/s40261-021-01001-0",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1371/journal.pone.0247122",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1038/s41586-020-2521-4",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1080/03610910902859574",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.1002/pds.1200",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"key": "ref49",
"unstructured": "Statement on the Management at Home or in a Care Facility of Suspected or Confirmed COVID-19 Patients\nhttps://www.hcsp.fr"
},
{
"DOI": "10.7326/0003-4819-147-8-200710160-00010",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.11613/BM.2021.010502",
"doi-asserted-by": "publisher",
"key": "ref51"
}
],
"reference-count": 51,
"references-count": 51,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2077-0383/10/24/5891"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Hydroxyzine Use and Mortality in Patients Hospitalized for COVID-19: A Multicenter Observational Study",
"type": "journal-article",
"volume": "10"
}
