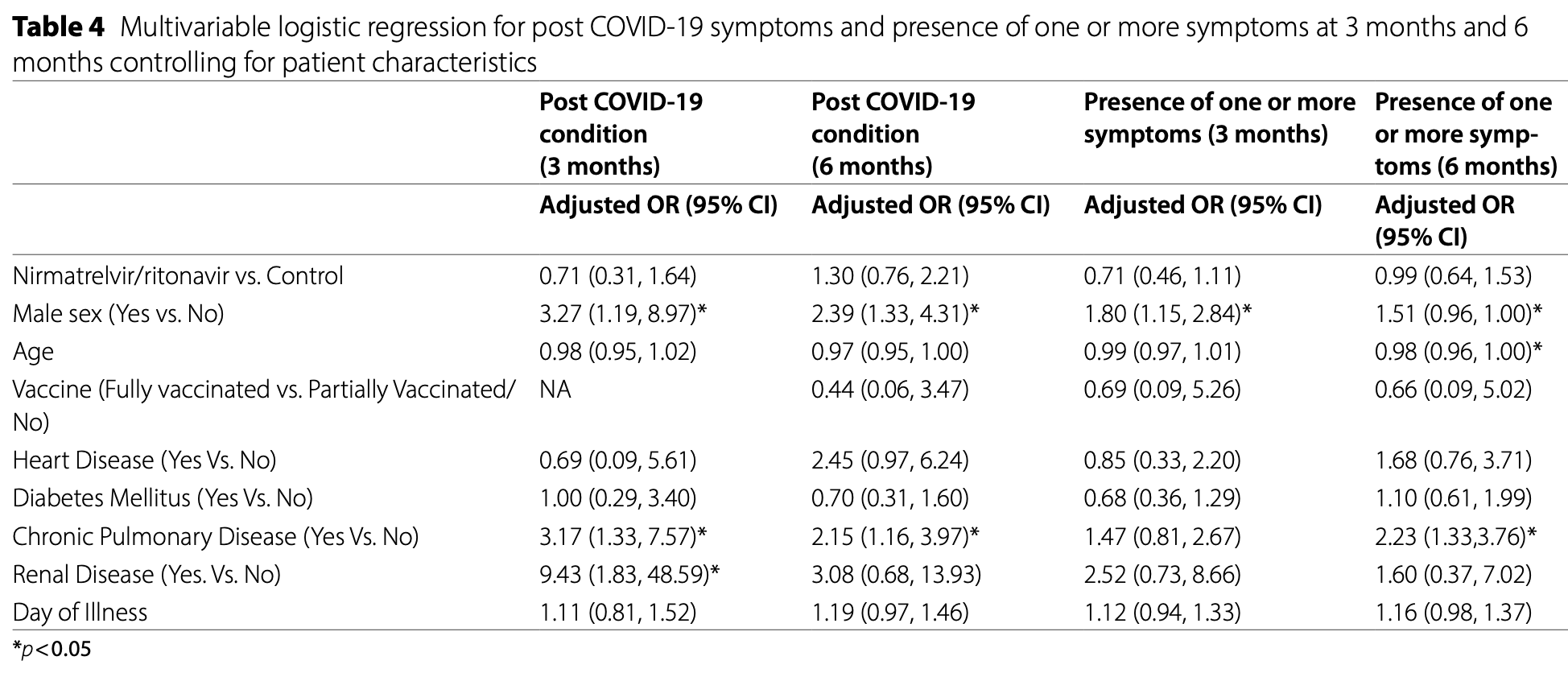
Nirmatrelvir/ritonavir treatment and the risk of post-COVID condition over 180 days in Malaysia
et al., BMC Infectious Diseases, doi:10.1186/s12879-024-09679-1, Aug 2024
Retrospective 2,524 adult COVID-19 outpatients in Malaysia showing no significant difference in post-COVID condition (PCC) at 3 months and 6 months with paxlovid treatment.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
|
risk of long COVID, 30.0% higher, OR 1.30, p = 0.34, treatment 1,289, control 1,235, adjusted per study, multivariable, day 180, RR approximated with OR.
|
|
risk of long COVID, 29.0% lower, OR 0.71, p = 0.43, treatment 1,289, control 1,235, adjusted per study, multivariable, day 90, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
Low et al., 5 Aug 2024, retrospective, Malaysia, peer-reviewed, 11 authors, study period 14 July, 2022 - 14 May, 2023.
Contact: evienlow@gmail.com, evlow@moh.gov.my.
Nirmatrelvir/ritonavir treatment and the risk of post-COVID condition over 180 days in Malaysia
BMC Infectious Diseases, doi:10.1186/s12879-024-09679-1
Background The effect of nirmatrelvir/ritonavir on preventing post-COVID condition (PCC) in the BA4, BA5, and XBB Omicron predominant periods is not well understood. The purpose of this study was to assess how nirmatrelvir/ ritonavir treatment affected both PCC and health-related quality of life. Methods This retrospective cohort study enrolled 2,524 adults aged 18 years and older who were eligible for nirmatrelvir/ritonavir between July 14 to November 14, 2022. All outcomes were observed from the patient's first visit to the primary health clinic, 1 week, 1 month, 3 months, and 6 months after testing positive for COVID-19. The primary outcome was the presence of PCC. Secondary outcomes included the effects on health-related quality of life, such as walking, bathing and dressing, activities, cause adverse emotions or signs that prevent individuals from leading normal lives over a 180-day observation period. Results There were no significant differences observed between the nirmatrelvir/ritonavir and those not administered (control group) in terms of PCC symptoms at 3 months (OR 0.71 95% CI 0.31, 1.64) and 6 months (OR 1.30 95% CI 0.76, 2.21). At 3 months, the use of nirmatrelvir/ritonavir was associated with a 26% reduction in symptoms causing negative emotions (OR 0.74 95% CI 0.60, 0.92) and an increased likelihood of symptoms limiting walking (OR 1.58 95% CI 1.10, 2.27). However, there were no significant differences between the nirmatrelvir/ritonavir and the control group in terms of the impact of PCC on health-related quality of life at 6 months.
Conclusions Our study indicates that the administration of nirmatrelvir/ritonavir does not significantly reduce PCC after 3 months and 6 months in a population with high vaccination coverage.
Supplementary Information The online version contains supplementary material available at https://doi. org/10.1186/s12879-024-09679-1.
Supplementary Material 1 Supplementary Material 2 Author contributions EVL, MDP and KP designed the study. WRK, WJL, MR and ZWT collected the data. EVL and YYT acquired and analyzed the data. The data was interpreted by all authors. EVL wrote the first draft of the manuscript. All authors reviewed and edited the manuscript. EVL, MD, SC, MI, AAS and KP performed the critical revision of the manuscript for intellectual content. All authors had full access to all data in the studies and had final responsibility for the decision to submit for publication.
Data availability The databases consist of individual-level information. The deidentified data that support the findings of this study are available upon reasonable request, and with protocol approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia.
Declarations Ethical approval This study was registered in the National Medical Research Register and approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia (NMRR ID-22-00938-2YN).
Data sharing statement The databases consist of individual-level information. The deidentified data that support the findings of this study are available upon reasonable request, and with protocol approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia.
Competing interests The authors..
References
Aggarwal, Molina, Beaty, Bennett, Carlson, Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study, The Lancet Infectious Diseases
Anand, PM Muhyiddin receives first Covid-19 vaccine as Malaysia kicks off mass innoculation campaign
Arbel, Sagy, Hoshen, Battat, Lavie et al., Nirmatrelvir use and severe Covid-19 outcomes during the Omicron Surge, N Engl J Med
Bajema, Berry, Streja, Rajeevan, Li et al., Effectiveness of COVID-19 Treatment with Nirmatrelvir-Ritonavir or Molnupiravir among U.S. veterans: Target Trial Emulation Studies with one-Month and Six-Month outcomes, Ann Intern Med
Congdon, Narrowe, Yone, Gunn, Deng et al., Nirmatrelvir/ritonavir and risk of long COVID symptoms: a retrospective cohort study, Sci Rep
Dryden, Mudara, Vika, Blumberg, Mayet et al., Post-COVID-19 condition 3 months after hospitalisation with SARS-CoV-2 in South Africa: a prospective cohort study, Lancet Glob Health
Hedberg, Nauclér, Post-COVID-19 Condition after SARS-CoV-2 infections during the Omicron Surge vs the Delta, Alpha, and wild type periods in Stockholm, Sweden, J Infect Dis
Lai, Wang, Chen, Chen, Wang, The clinical efficacy and safety of anti-viral agents for non-hospitalized patients with COVID-19: a systematic review and network Meta-analysis of Randomized controlled trials, Viruses
Lewnard, Mclaughlin, Malden, Hong, Puzniak et al., Effectiveness of nirmatrelvir-ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system, The Lancet Infectious Diseases
Liu, Wu, Huang, Tsai, Lai, The effect of nirmatrelvir-ritonavir on the long-term risk of neuropsychiatric sequelae following COVID-19, J Med Virol
Low, Pathmanathan, Chidambaram, Kim, Lee et al., Real-world nirmatrelvir-ritonavir outpatient treatment in reducing hospitalization for high-risk patients with COVID-19 during Omicron BA.4, BA.5 and XBB subvariants dominance in Malaysia: a retrospective cohort study, Int J Infect Dis
Magnusson, Kristoffersen, Dell'isola, Kiadaliri, Turkiewicz et al., Post-covid medical complaints following infection with SARS-CoV-2 omicron vs Delta variants, Nat Commun
Marzi, Vakil, Bahmanyar, Zarenezhad, Mechanism of action, synthesis, and in Silico Study, Biomed Res Int
Mohammed, Nauman, Paul, Ganesan, Chen et al., The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: a systematic review, Hum Vaccin Immunother
Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of Paxlovid in reducing severe coronavirus Disease 2019 and mortality in high-risk patients, Clin Infect Dis
O'mahoney, Routen, Gillies, Ekezie, Welford et al., The prevalence and long-term health effects of long covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis, EClinicalMedicine
Pbkd, SENARAI PRODUK -PRODUK YANG TELAH DILULUSKAN OLEH PIHAK BERKUASA KAWALAN DADAH
Saunders, Sperling, Bendstrup, A new paradigm is needed to explain long COVID, Lancet Respiratory Med
Sim, Chidambaram, Wong, Pathmanathan, Peariasamy et al., Clinical characteristics and risk factors for severe COVID-19 infections in Malaysia: a nationwide observational study, Lancet Reg Health West Pac
Tok, Kang, Ng, Rahman, Syahmi et al., Post COVID-19 condition among adults in Malaysia following the Omicron wave: a prospective cohort study, PLoS ONE
Von Elm, Altman, Egger, Pocock, Gøtzsche et al., The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies, Lancet
Wai, Chan, Cheung, Wang, Chan et al., Association of Molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19, Lancet Reg Health -Western Pac
Wong, Lau, Leung, Real-world effectiveness of nirmatrelvir/ritonavir against BA.4 and BA.5 omicron SARS-CoV-2 variants, Lancet Infect Dis
Woodrow, Carey, Ziauddeen, Thomas, Akrami et al., Systematic review of the prevalence of long COVID, Open Forum Infect Dis
Xie, Choi, Al-Aly, Association of Treatment with Nirmatrelvir and the risk of Post-COVID-19 Condition, JAMA Internal Medicine
DOI record:
{
"DOI": "10.1186/s12879-024-09679-1",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-024-09679-1",
"alternative-id": [
"9679"
],
"article-number": "780",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "31 May 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "29 July 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "5 August 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethical approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "This study was registered in the National Medical Research Register and approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia (NMRR ID-22-00938-2YN)."
},
{
"group": {
"label": "Data sharing statement",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The databases consist of individual-level information. The deidentified data that support the findings of this study are available upon reasonable request, and with protocol approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 5,
"value": "The authors declare that they have no conflict of interests."
}
],
"author": [
{
"affiliation": [],
"family": "Low",
"given": "Ee Vien",
"sequence": "first"
},
{
"affiliation": [],
"family": "Pathmanathan",
"given": "Mohan Dass",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ten",
"given": "Yi Yang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chidambaram",
"given": "Suresh Kumar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kim",
"given": "Wee Ric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Wei Jia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teh",
"given": "Zhi Wei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Appannan",
"given": "Maheshwara Rao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ismail",
"given": "Mastura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Samad",
"given": "Azah Abdul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peariasamy",
"given": "Kalaiarasu M.",
"sequence": "additional"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
8,
5
]
],
"date-time": "2024-08-05T06:03:12Z",
"timestamp": 1722837792000
},
"deposited": {
"date-parts": [
[
2024,
8,
5
]
],
"date-time": "2024-08-05T06:03:53Z",
"timestamp": 1722837833000
},
"funder": [
{
"award": [
"NIH/800-3/2/2 Jilid 13 (182)"
],
"name": "Ministry of Health Malaysia Research Grant"
}
],
"indexed": {
"date-parts": [
[
2024,
8,
6
]
],
"date-time": "2024-08-06T00:22:25Z",
"timestamp": 1722903745226
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
8,
5
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
5
]
],
"date-time": "2024-08-05T00:00:00Z",
"timestamp": 1722816000000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
5
]
],
"date-time": "2024-08-05T00:00:00Z",
"timestamp": 1722816000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-024-09679-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-024-09679-1/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-024-09679-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
8,
5
]
]
},
"published-online": {
"date-parts": [
[
2024,
8,
5
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "9679_CR1",
"unstructured": "Ministry of Health Malaysia. COVID-19: What is latest situation in Malaysia? 2024 https://data.moh.gov.my/dashboard/covid-19. Accessed 15 Dec 2023."
},
{
"DOI": "10.1080/21645515.2022.2027160",
"author": "I Mohammed",
"doi-asserted-by": "publisher",
"first-page": "2027160",
"issue": "1",
"journal-title": "Hum Vaccin Immunother",
"key": "9679_CR2",
"unstructured": "Mohammed I, Nauman A, Paul P, Ganesan S, Chen KH, Jalil SMS, et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: a systematic review. Hum Vaccin Immunother. 2022;18(1):2027160.",
"volume": "18",
"year": "2022"
},
{
"key": "9679_CR3",
"unstructured": "Centers for Disease Control and Prevention. Benefits of Getting https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-benefits.html. Accessed 15 Dec 2023."
},
{
"key": "9679_CR4",
"unstructured": "Anand R. PM Muhyiddin receives first Covid-19 vaccine as Malaysia kicks off mass innoculation campaign. Straits Time. 2021."
},
{
"key": "9679_CR5",
"unstructured": "Ministry of Health Malaysia. COVIDNOW: Vaccination Progress 2023. https://covidnow.moh.gov.my/vaccinations/?refresh=1720781109523. Accessed 1 January 2024."
},
{
"author": "BLH Sim",
"first-page": "100055",
"journal-title": "Lancet Reg Health West Pac",
"key": "9679_CR6",
"unstructured": "Sim BLH, Chidambaram SK, Wong XC, Pathmanathan MD, Peariasamy KM, Hor CP, et al. Clinical characteristics and risk factors for severe COVID-19 infections in Malaysia: a nationwide observational study. Lancet Reg Health West Pac. 2020;4:100055.",
"volume": "4",
"year": "2020"
},
{
"key": "9679_CR7",
"unstructured": "Centers for Disease Control and Prevention. Underlying Medical Conditions 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. Accessed 15 Dec 2023."
},
{
"DOI": "10.1016/S2214-109X(22)00286-8",
"author": "M Dryden",
"doi-asserted-by": "publisher",
"first-page": "e1247",
"issue": "9",
"journal-title": "Lancet Glob Health",
"key": "9679_CR8",
"unstructured": "Dryden M, Mudara C, Vika C, Blumberg L, Mayet N, Cohen C, et al. Post-COVID-19 condition 3 months after hospitalisation with SARS-CoV-2 in South Africa: a prospective cohort study. Lancet Glob Health. 2022;10(9):e1247–56.",
"volume": "10",
"year": "2022"
},
{
"key": "9679_CR9",
"unstructured": "Centers for Disease Control and Prevention, Long COVID. 2023. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html. Accessed 15 Dec 2023."
},
{
"DOI": "10.1016/j.eclinm.2022.101762",
"author": "LL O’Mahoney",
"doi-asserted-by": "publisher",
"first-page": "101762",
"journal-title": "EClinicalMedicine",
"key": "9679_CR10",
"unstructured": "O’Mahoney LL, Routen A, Gillies C, Ekezie W, Welford A, Zhang A, et al. The prevalence and long-term health effects of long covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. EClinicalMedicine. 2023;55:101762.",
"volume": "55",
"year": "2023"
},
{
"key": "9679_CR11",
"unstructured": "National Istitue for Health and Care Excellence (NICE). Royal College of General Practitioner (RCGP); Scottish intercollegiate Guidelines Network (SIGN); COVID-19 rapid guidelines: managing the long term effects of COVID-19. 2022. Accessed 15 Dec 2023."
},
{
"key": "9679_CR12",
"unstructured": "Government of Canada. Post COVID-19 condition (long COVID) 2023 [updated March 9. 2023. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/symptoms/post-covid-19-condition.html. Accessed 15 Dec 2023."
},
{
"key": "9679_CR13",
"unstructured": "Department of Health and Aged Care, Long COVID. 2023 https://www.health.gov.au/topics/covid-19/long-covid. Accessed 15 Dec 2023."
},
{
"DOI": "10.1093/ofid/ofad233",
"author": "M Woodrow",
"doi-asserted-by": "publisher",
"first-page": "ofad233",
"issue": "7",
"journal-title": "Open Forum Infect Dis",
"key": "9679_CR14",
"unstructured": "Woodrow M, Carey C, Ziauddeen N, Thomas R, Akrami A, Lutje V, et al. Systematic review of the prevalence of long COVID. Open Forum Infect Dis. 2023;10(7):ofad233.",
"volume": "10",
"year": "2023"
},
{
"key": "9679_CR15",
"unstructured": "FDA. FDA Approves First Oral Antiviral for Treatment of COVID-19 in Adults. 2023 https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-antiviral-treatment-covid-19-adults. Accessed 15 Nov 2023."
},
{
"key": "9679_CR16",
"unstructured": "Aggarwal NR, Molina KC, Beaty LE, Bennett TD, Carlson NE, Mayer DA et al. Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study. The Lancet Infectious Diseases."
},
{
"DOI": "10.1056/NEJMoa2204919",
"author": "R Arbel",
"doi-asserted-by": "publisher",
"first-page": "790",
"issue": "9",
"journal-title": "N Engl J Med",
"key": "9679_CR17",
"unstructured": "Arbel R, Wolff Sagy Y, Hoshen M, Battat E, Lavie G, Sergienko R, et al. Nirmatrelvir use and severe Covid-19 outcomes during the Omicron Surge. N Engl J Med. 2022;387(9):790–8.",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac443",
"author": "R Najjar-Debbiny",
"doi-asserted-by": "publisher",
"first-page": "e342",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "9679_CR18",
"unstructured": "Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, et al. Effectiveness of Paxlovid in reducing severe coronavirus Disease 2019 and mortality in high-risk patients. Clin Infect Dis. 2022;76(3):e342–9.",
"volume": "76",
"year": "2022"
},
{
"DOI": "10.1016/j.lanwpc.2022.100602",
"doi-asserted-by": "crossref",
"key": "9679_CR19",
"unstructured": "Wai AK-C, Chan CY, Cheung AW-L, Wang K, Chan SC-L, Lee TT-L et al. Association of Molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. Lancet Reg Health – Western Pac. 2023;30."
},
{
"DOI": "10.1155/2022/7341493",
"doi-asserted-by": "crossref",
"key": "9679_CR20",
"unstructured": "Marzi M, Vakil MK, Bahmanyar M, Zarenezhad E, Paxlovid. Mechanism of action, synthesis, and in Silico Study. Biomed Res Int. 2022;2022:7341493."
},
{
"DOI": "10.1038/s41598-023-46912-4",
"author": "S Congdon",
"doi-asserted-by": "publisher",
"first-page": "19688",
"issue": "1",
"journal-title": "Sci Rep",
"key": "9679_CR21",
"unstructured": "Congdon S, Narrowe Z, Yone N, Gunn J, Deng Y, Nori P, et al. Nirmatrelvir/ritonavir and risk of long COVID symptoms: a retrospective cohort study. Sci Rep. 2023;13(1):19688.",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1001/jamainternmed.2023.0743",
"doi-asserted-by": "crossref",
"key": "9679_CR22",
"unstructured": "Xie Y, Choi T, Al-Aly Z. Association of Treatment with Nirmatrelvir and the risk of Post–COVID-19 Condition. JAMA Internal Medicine; 2023."
},
{
"key": "9679_CR23",
"unstructured": "National Pharmaceutical Regulatory Agency. SENARAI PRODUK – PRODUK YANG TELAH DILULUSKAN OLEH PIHAK BERKUASA KAWALAN DADAH (PBKD.) DALAM MESYUARAT PBKD KALI KE – 370. 2022."
},
{
"DOI": "10.1016/S1473-3099(23)00118-4",
"doi-asserted-by": "crossref",
"key": "9679_CR24",
"unstructured": "Lewnard JA, McLaughlin JM, Malden D, Hong V, Puzniak L, Ackerson BK, et al. Effectiveness of nirmatrelvir-ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system. The Lancet Infectious Diseases; 2023."
},
{
"DOI": "10.3390/v14081706",
"doi-asserted-by": "crossref",
"key": "9679_CR25",
"unstructured": "Lai CC, Wang YH, Chen KH, Chen CH, Wang CY. The clinical efficacy and safety of anti-viral agents for non-hospitalized patients with COVID-19: a systematic review and network Meta-analysis of Randomized controlled trials. Viruses. 2022;14(8)."
},
{
"DOI": "10.1016/S1473-3099(23)00056-7",
"author": "CKH Wong",
"doi-asserted-by": "publisher",
"first-page": "639",
"issue": "6",
"journal-title": "Lancet Infect Dis",
"key": "9679_CR26",
"unstructured": "Wong CKH, Lau KTK, Leung GM. Real-world effectiveness of nirmatrelvir/ritonavir against BA.4 and BA.5 omicron SARS-CoV-2 variants. Lancet Infect Dis. 2023;23(6):639–40.",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/j.ijid.2023.08.003",
"author": "EV Low",
"doi-asserted-by": "publisher",
"first-page": "77",
"journal-title": "Int J Infect Dis",
"key": "9679_CR27",
"unstructured": "Low EV, Pathmanathan MD, Chidambaram SK, Kim WR, Lee WJ, Teh ZW, et al. Real-world nirmatrelvir-ritonavir outpatient treatment in reducing hospitalization for high-risk patients with COVID-19 during Omicron BA.4, BA.5 and XBB subvariants dominance in Malaysia: a retrospective cohort study. Int J Infect Dis. 2023;135:77–83.",
"volume": "135",
"year": "2023"
},
{
"DOI": "10.7326/M22-3565",
"author": "KL Bajema",
"doi-asserted-by": "publisher",
"first-page": "807",
"issue": "6",
"journal-title": "Ann Intern Med",
"key": "9679_CR28",
"unstructured": "Bajema KL, Berry K, Streja E, Rajeevan N, Li Y, Mutalik P, et al. Effectiveness of COVID-19 Treatment with Nirmatrelvir-Ritonavir or Molnupiravir among U.S. veterans: Target Trial Emulation Studies with one-Month and Six-Month outcomes. Ann Intern Med. 2023;176(6):807–16.",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(07)61602-X",
"author": "E von Elm",
"doi-asserted-by": "publisher",
"first-page": "1453",
"issue": "9596",
"journal-title": "Lancet",
"key": "9679_CR29",
"unstructured": "von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7.",
"volume": "370",
"year": "2007"
},
{
"key": "9679_CR30",
"unstructured": "Ministry of Health Malaysia. Clinical Management of Confirmed COVID-19 Case in Adult and Paediatric 2022. https://covid-19.moh.gov.my/garis-panduan/garis-panduan-kkm/ANNEX-2E-CLINICAL-MANAGEMENT-OF-CONFIRMED-COVID-19-31052022.pdf. Accessed 15 Nov 2023."
},
{
"DOI": "10.1371/journal.pone.0296488",
"author": "PSK Tok",
"doi-asserted-by": "publisher",
"first-page": "e0296488",
"issue": "1",
"journal-title": "PLoS ONE",
"key": "9679_CR31",
"unstructured": "Tok PSK, Kang KY, Ng SW, Ab Rahman N, Syahmi MA, Pathmanathan MD, et al. Post COVID-19 condition among adults in Malaysia following the Omicron wave: a prospective cohort study. PLoS ONE. 2024;19(1):e0296488.",
"volume": "19",
"year": "2024"
},
{
"key": "9679_CR32",
"unstructured": "World Health Organization. Post COVID-19 condition (COVID-19) 2022 https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition. Accessed 15 November 2023."
},
{
"DOI": "10.1038/s41467-022-35240-2",
"author": "K Magnusson",
"doi-asserted-by": "publisher",
"first-page": "7363",
"issue": "1",
"journal-title": "Nat Commun",
"key": "9679_CR33",
"unstructured": "Magnusson K, Kristoffersen DT, Dell’Isola A, Kiadaliri A, Turkiewicz A, Runhaar J, et al. Post-covid medical complaints following infection with SARS-CoV-2 omicron vs Delta variants. Nat Commun. 2022;13(1):7363.",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiad382",
"author": "P Hedberg",
"doi-asserted-by": "publisher",
"first-page": "133",
"issue": "1",
"journal-title": "J Infect Dis",
"key": "9679_CR34",
"unstructured": "Hedberg P, Nauclér P. Post-COVID-19 Condition after SARS-CoV-2 infections during the Omicron Surge vs the Delta, Alpha, and wild type periods in Stockholm, Sweden. J Infect Dis. 2024;229(1):133–6.",
"volume": "229",
"year": "2024"
},
{
"DOI": "10.1016/S2213-2600(22)00501-X",
"author": "C Saunders",
"doi-asserted-by": "publisher",
"first-page": "e12",
"issue": "2",
"journal-title": "Lancet Respiratory Med",
"key": "9679_CR35",
"unstructured": "Saunders C, Sperling S, Bendstrup E. A new paradigm is needed to explain long COVID. Lancet Respiratory Med. 2023;11(2):e12–3.",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28951",
"author": "TH Liu",
"doi-asserted-by": "publisher",
"first-page": "e28951",
"issue": "7",
"journal-title": "J Med Virol",
"key": "9679_CR36",
"unstructured": "Liu TH, Wu JY, Huang PY, Tsai YW, Lai CC. The effect of nirmatrelvir-ritonavir on the long-term risk of neuropsychiatric sequelae following COVID-19. J Med Virol. 2023;95(7):e28951.",
"volume": "95",
"year": "2023"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-024-09679-1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Nirmatrelvir/ritonavir treatment and the risk of post-COVID condition over 180 days in Malaysia",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "24"
}