Repurposing Colchicine in Treating Patients with COVID-19: A Systematic Review and Meta-Analysis
et al., Life, doi:10.3390/life11080864, Aug 2021
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
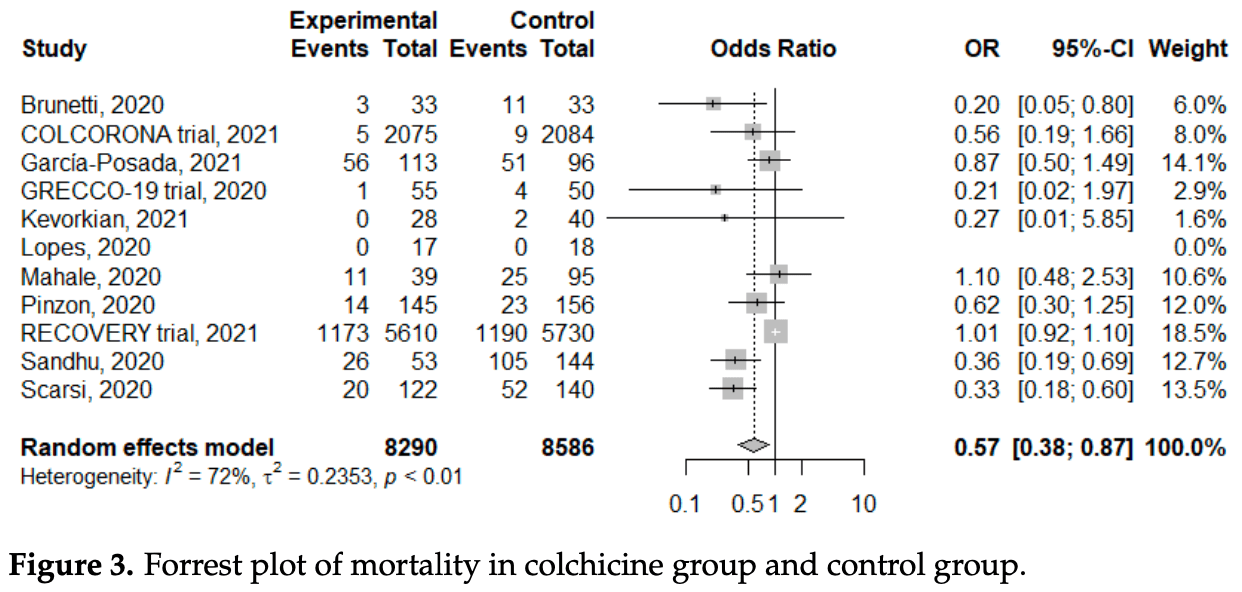
Meta analysis of 11 colchicine studies showing significantly lower mortality with treatment.
10 meta-analyses show significant improvements with colchicine for mortality1-8,
oxygen therapy8,
hospitalization9, and
severity10.
Currently there are 54 colchicine for COVID-19 studies, showing 22% lower mortality [12‑31%], 29% lower ventilation [-15‑56%], 34% lower ICU admission [8‑52%], 17% lower hospitalization [9‑25%], and 9% more cases [-8‑29%].
|
risk of death, 43.0% lower, OR 0.57, p = 0.008, RR approximated with OR.
|
|
risk of mechanical ventilation, 33.0% lower, OR 0.67, p = 0.15, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zein et al., Effect of colchicine on mortality in patients with COVID-19 – A systematic review and meta-analysis, Diabetes & Metabolic Syndrome: Clinical Research & Reviews, doi:10.1016/j.dsx.2022.102395.
2.
Rai et al., The Potential Role of Colchicine in Reducing Mortality and Mechanical Ventilation Rates in COVID-19 Infection: A Meta-analysis, Journal of Advances in Medicine and Medical Research, doi:10.9734/jammr/2022/v34i2031503.
3.
Elshafei et al., Colchicine use might be associated with lower mortality in COVID‐19 patients: A meta‐analysis, European Journal of Clinical Investigation, doi:10.1111/eci.13645.
4.
Lien et al., Repurposing Colchicine in Treating Patients with COVID-19: A Systematic Review and Meta-Analysis, Life, doi:10.3390/life11080864.
5.
Danjuma et al., Does Colchicine Reduce Mortality in Patients with Covid-19 Clinical Syndrome? An Umbrella Review of Published Meta-Analyses, Elsevier BV, doi:10.2139/ssrn.4447127.
6.
Salah et al., Meta-analysis of the Effect of Colchicine on Mortality and Mechanical Ventilation in COVID-19, The American Journal of Cardiology, doi:10.1016/j.amjcard.2021.02.005.
7.
Golpour et al., The effectiveness of Colchicine as an anti-inflammatory drug in the treatment of coronavirus disease 2019: Meta-analysis, International Journal of Immunopathology and Pharmacology, doi:10.1177/20587384211031763.
8.
Elshiwy et al., The role of colchicine in the management of COVID-19: a meta-analysis, BMC Pulmonary Medicine, doi:10.1186/s12890-024-03001-0.
Lien et al., 23 Aug 2021, peer-reviewed, 12 authors.
Contact: mmhped.lin@gmail.com (corresponding author), 4976@mmh.org.tw, 4554@mmh.org.tw, 4467@mmh.org.tw, 3099@mmh.org.tw, drlawrenceliu@gmail.com, superlof@gmail.com, lazyleisure@gmail.com, chi.4531@mmh.org.tw, ncc88@mmh.org.tw, mento1218@gmail.com, yvon1207@gmail.com.
Repurposing Colchicine in Treating Patients with COVID-19: A Systematic Review and Meta-Analysis
Life, doi:10.3390/life11080864
Coronavirus disease 2019 (COVID-19) had caused huge health losses worldwide. Several drugs had been applied to treat patients with COVID-19, and repurposing colchicine had been proposed for its anti-inflammatory properties via several pathways. In this systematic review, we evaluated the effects of colchicine treatment. From inception to May 31, 2021, databases, including PubMed, EMbase, medRxiv, and Research Square were searched, and 11 studies were enrolled. A total of 17,205 COVID-19 patients with male predominance (62.9%) were analyzed. Patients with colchicine treatment had a significantly lower risk of mortality (odds ratio (OR): 0.57, 95% confidence interval (CI): 0.38-0.87, I 2 : 72%; p < 0.01) and a non-significantly lower rate of mechanical ventilation (OR: 0.67, 95%CI: 0.39-1.15). The side effects were mild and not significantly different (OR: 2.03, 95%CI: 0.51-8.09). Subgroup analysis with randomized controlled trials showed no statistically significant difference in the mortality (OR: 0.80, 95%CI: 0.44-1.46, I 2 : 33%; p = 0.22). In conclusion, our meta-analysis found that colchicine treatment was associated with a significantly lower risk of mortality in patients with COVID-19. However, this benefit was not observed in the subgroup analysis of randomized controlled trials. Further randomized controlled studies are required to confirm the potential benefits of colchicine treatment.
Conclusions In conclusion, our systematic review and meta-analysis identified 17,205 COVID-19 patients, and we found that a significant reduction in mortality in patients with colchicine treatment (OR: 0.57). The results were similar with subgroup analysis for different mortality rates. However, moderate heterogeneity was observed, and the dose, interval, duration, and mortality rate varied across studies. Further subgroup analysis with randomized controlled trials showed a non-significant decrease. Funnel plots and Egger's test demonstrated a significant publication bias in both meta-analysis of all studies and randomized controlled trials. Therefore, further well-designed randomized controlled trials were required to elucidate the benefits of colchicine treatment and determine the optimal regimen. Although colchicine was cheap, easily available, accessible, and safe, routine colchicine treatment was not recommended based on our systematic review and meta-analysis.
Supplementary Materials: The following are available online at https://www.mdpi.com/article/10 .3390/life11080864/s1, Supplementary File S1, Complete search strategy of our systematic review. Supplementary File S2, Funnel plot of enrolled studies investigating the subsequent mortality of colchicine and control groups. Supplementary File S3, Contour-enhanced funnel plot of enrolled studies investigating subsequent mortality of colchicine and control groups. Supplementary File S4, Egger's test of enrolled studies..
References
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of COVID-19-Final report, N. Engl. J. Med, doi:10.1056/NEJMoa2007764
Brunetti, Diawara, Tsai, Firestein, Nahass et al., Colchicine to weather the cytokine storm in hospitalized patients with COVID-19, J. Clin. Med, doi:10.3390/jcm9092961
Chen, Tseng, Choi, Lee, Su et al., Taiwan government-guided strategies contributed to combating and controlling COVID-19 pandemic, Front. Public Health
Chi, Chiu, Peng, Lin, Tai et al., One-seventh of patients with COVID-19 had olfactory and gustatory abnormalities as their initial symptoms: A systematic review and meta-analysis, Life, doi:10.3390/life10090158
Chi, Chiu, Tai, Chang, Lin et al., Clinical features of neonates born to mothers with coronavirus disease-2019: A ssytematic review of 105 neonates, J. Microbiol. Immunol. Infect
Dalbeth, Lauterio, Wolfe, Mechanism of action of colchicine in the treatment of gout, Clin. Ther
De Rivero Vaccari, Dietrich, Keane, De Rivero, Vaccari, The inflammasome in times of COVID-19, Front. Immunol, doi:10.3389/fimmu.2020.583373
Deftereos, Giannopoulos, Vrachatis, Siasos, Giotaki et al., Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: The grecco-19 randomized clinical trial, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2020.13136
Dupuis, Sirois, Rhéaume, Nguyen, Clavet-Lanthier et al., Colchicine reduces lung injury in experimental acute respiratory distress syndrome, PLoS ONE, doi:10.1371/journal.pone.0242318
Egger, Smith, Schneider, Minder, Bias in meta-analysis detected by a simple, graphical test, BMJ, doi:10.1136/bmj.315.7109.629
Fajgenbaum, June, Cytokine storm, N. Engl. J. Med, doi:10.1056/NEJMra2026131
Freeman, Swartz, Targeting the nlrp3 inflammasome in severe COVID-19, Front. Immunol, doi:10.3389/fimmu.2020.01518
García-Posada, Aruachan-Vesga, Mestra, Humánez, Serrano-Coll et al., Clinical outcomes of patients hospitalized for COVID-19 and evidence-based on the pharmacological management reduce mortality in a region of the colombian caribbean, J. Infect. Public Health, doi:10.1016/j.jiph.2021.02.013
Hariyanto, Halim, Jodhinata, Yanto, Kurniawan, Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis, Clin. Exp. Pharm. Physiol, doi:10.1111/1440-1681.13488
Higgins, Thomas, Chandler, Cumpston, Li et al., Cochrane Handbook for Systematic Reviews Ofinterventions Version
Higgins, Thompson, Deeks, Altman, Measuring inconsistency in meta-analyses, BMJ
Horby, Campbell, Spata, Emberson, Staplin et al., COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial, doi:10.1101/2021.05.18.21257267
Kevorkian, Lopes, Sène, Riveline, Vandiedonck et al., Oral corticoid, aspirin, anticoagulant, colchicine, and furosemide to improve the outcome of hospitalized COVID-19 patients-The cocaa-cola cohort study, J. Infect, doi:10.1016/j.jinf.2021.02.008
Kowalewski, Fina, Słomka, Raffa, Martucci et al., Covid-19 and ecmo: The interplay between coagulation and inflammation-A narrative review, Crit. Care
Leung, Yao Hui, Kraus, Colchicine-Update on mechanisms of action and therapeutic uses, Semin. Arthritis Rheum, doi:10.1016/j.semarthrit.2015.06.013
Lopes, Bonjorno, Giannini, Amaral, Menezes et al., Beneficial effects of colchicine for moderate to severe COVID-19: A randomised, double-blinded, placebo-controlled clinical trial, RMD Open, doi:10.1136/rmdopen-2020-001455
Mahale, Rajhans, Godavarthy, Narasimhan, Oak et al., A retrospective observational study of hypoxic COVID-19 patients treated with immunomodulatory drugs in a tertiary care hospital, Indian J. Crit. Care Med
Martínez, Celermajer, Patel, The nlrp3 inflammasome and the emerging role of colchicine to inhibit atherosclerosisassociated inflammation, Atherosclerosis, doi:10.1016/j.atherosclerosis.2017.12.027
Martínez, Robertson, Barraclough, Xia, Mallat et al., Colchicine acutely suppresses local cardiac production of inflammatory cytokines in patients with an acute coronary syndrome, J. Am. Heart Assoc, doi:10.1161/JAHA.115.002128
Page, Mckenzie, Bossuyt, Boutron, Hoffmann et al., The prisma 2020 statement: An updated guideline for reporting systematic reviews, BMJ
Pinzón, Arango, Betancur, Holguín, Arias et al., Clinical outcome of patients with COVID-19 pneumonia treated with corticosteroids and colchicine in colombia, ResearchSquare
Rabaan, Al-Ahmed, Garout, Al-Qaaneh, Sule et al., Diverse immunological factors influencing pathogenesis in patients with COVID-19: A review on viral dissemination, immunotherapeutic options to counter cytokine storm and inflammatory responses, Pathogens, doi:10.3390/pathogens10050565
Sandhu, Tieng, Chilimuri, Franchin, A case control study to evaluate the impact of colchicine on patients admitted to the hospital with moderate to severe COVID-19 infection, Can. J. Infect. Dis. Med. Microbiol, doi:10.1155/2020/8865954
Scarsi, Piantoni, Colombo, Airó, Richini et al., Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome, Ann. Rheum. Dis, doi:10.1136/annrheumdis-2020-217712
Schlesinger, Firestein, Brunetti, Colchicine in COVID-19: An old drug, new use, Curr. Pharmacol. Rep, doi:10.1007/s40495-020-00225-6
Shah, Novel coronavirus-induced nlrp3 inflammasome activation: A potential drug target in the treatment of COVID-19, Front. Immunol, doi:10.3389/fimmu.2020.01021
Siemieniuk, Bartoszko, Ge, Zeraatkar, Izcovich et al., Drug treatments for COVID-19: Living systematic review and network meta-analysis, BMJ
Sterne, Murthy, Diaz, Slutsky, Villar et al., Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis, JAMA
Stewart, Yang, Atkins, Dalbeth, Robinson, Adverse events during oral colchicine use: A systematic review and meta-analysis of randomised controlled trials, Arthritis Res. Ther, doi:10.1186/s13075-020-2120-7
Swanson, Deng, Ting, The nlrp3 inflammasome: Molecular activation and regulation to therapeutics, Nat. Rev. Immunol, doi:10.1038/s41577-019-0165-0
Tardif, Bouabdallaoui, L'allier, Gaudet, Shah et al., Colchicine for community-treated patients with COVID-19 (COLCORONA): A phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial, Lancet Respir. Med, doi:10.1016/S2213-2600(21)00222-8
Van Den Berg, Velde, Severe COVID-19: Nlrp3 inflammasome dysregulated, Front. Immunol, doi:10.3389/fimmu.2020.01580
Wells, Shea, O'connell, The Newcastle-Ottawa Scale (nos) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses
Yuki, Fujiogi, Koutsogiannaki, COVID-19 pathophysiology: A review, Clin. Immunol, doi:10.1016/j.clim.2020.108427
DOI record:
{
"DOI": "10.3390/life11080864",
"ISSN": [
"2075-1729"
],
"URL": "http://dx.doi.org/10.3390/life11080864",
"abstract": "<jats:p>Coronavirus disease 2019 (COVID-19) had caused huge health losses worldwide. Several drugs had been applied to treat patients with COVID-19, and repurposing colchicine had been proposed for its anti-inflammatory properties via several pathways. In this systematic review, we evaluated the effects of colchicine treatment. From inception to May 31, 2021, databases, including PubMed, EMbase, medRxiv, and Research Square were searched, and 11 studies were enrolled. A total of 17,205 COVID-19 patients with male predominance (62.9%) were analyzed. Patients with colchicine treatment had a significantly lower risk of mortality (odds ratio (OR): 0.57, 95% confidence interval (CI): 0.38–0.87, I2: 72%; p < 0.01) and a non-significantly lower rate of mechanical ventilation (OR: 0.67, 95%CI: 0.39–1.15). The side effects were mild and not significantly different (OR: 2.03, 95%CI: 0.51–8.09). Subgroup analysis with randomized controlled trials showed no statistically significant difference in the mortality (OR: 0.80, 95%CI: 0.44–1.46, I2: 33%; p = 0.22). In conclusion, our meta-analysis found that colchicine treatment was associated with a significantly lower risk of mortality in patients with COVID-19. However, this benefit was not observed in the subgroup analysis of randomized controlled trials. Further randomized controlled studies are required to confirm the potential benefits of colchicine treatment.</jats:p>",
"alternative-id": [
"life11080864"
],
"author": [
{
"affiliation": [],
"family": "Lien",
"given": "Chi-Hone",
"sequence": "first"
},
{
"affiliation": [],
"family": "Lee",
"given": "Ming-Dar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Weng",
"given": "Shun-Long",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5591-0907",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lin",
"given": "Chao-Hsu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Lawrence Yu-Min",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0433-1072",
"affiliation": [],
"authenticated-orcid": false,
"family": "Tai",
"given": "Yu-Lin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lei",
"given": "Wei-Te",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3868-085X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Liu",
"given": "Jui-Ming",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9843-5189",
"affiliation": [],
"authenticated-orcid": false,
"family": "Huang",
"given": "Ya-Ning",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2385-344X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chi",
"given": "Hsin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3966-5021",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chiu",
"given": "Nan-Chang",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4630-8724",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lin",
"given": "Chien-Yu",
"sequence": "additional"
}
],
"container-title": "Life",
"container-title-short": "Life",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
8,
23
]
],
"date-time": "2021-08-23T14:24:17Z",
"timestamp": 1629728657000
},
"deposited": {
"date-parts": [
[
2021,
8,
26
]
],
"date-time": "2021-08-26T06:39:17Z",
"timestamp": 1629959957000
},
"indexed": {
"date-parts": [
[
2023,
2,
17
]
],
"date-time": "2023-02-17T21:30:50Z",
"timestamp": 1676669450886
},
"is-referenced-by-count": 15,
"issue": "8",
"issued": {
"date-parts": [
[
2021,
8,
23
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2021,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
8,
23
]
],
"date-time": "2021-08-23T00:00:00Z",
"timestamp": 1629676800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2075-1729/11/8/864/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "864",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
8,
23
]
]
},
"published-online": {
"date-parts": [
[
2021,
8,
23
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref1",
"unstructured": "Mortality Risk of COVID-19\nhttps://ourworldindata.org/mortality-risk-COVID"
},
{
"key": "ref2",
"unstructured": "Mortality Analyses\nhttps://coronavirus.jhu.edu/data/mortality"
},
{
"DOI": "10.3389/fpubh.2020.547423",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1016/j.clim.2020.108427",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1056/NEJMra2026131",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.3390/pathogens10050565",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1136/bmj.m2980",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1001/jama.2020.17023",
"article-title": "Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis",
"author": "Sterne",
"doi-asserted-by": "crossref",
"first-page": "1330",
"journal-title": "JAMA",
"key": "ref9",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/j.clinthera.2014.07.017",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1016/j.semarthrit.2015.06.013",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1186/s13075-020-2120-7",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1007/s40495-020-00225-6",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1371/journal.pone.0242318",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1111/1440-1681.13488",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1101/2021.05.18.21257267",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1016/S2213-2600(21)00222-8",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"article-title": "The prisma 2020 statement: An updated guideline for reporting systematic reviews",
"author": "Page",
"journal-title": "BMJ",
"key": "ref18",
"volume": "372",
"year": "2021"
},
{
"DOI": "10.3390/life10090158",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"key": "ref20",
"unstructured": "Cochrane Handbook for Systematic Reviews Ofinterventions Version 5.1.0 [updated march 2011]\nhttps://handbook-5-1.cochrane.org/"
},
{
"key": "ref21",
"unstructured": "The Newcastle-Ottawa Scale (nos) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses\nhttp://www.ohri.ca/programs/clinical_epidemiology/oxford.asp"
},
{
"DOI": "10.1136/bmj.327.7414.557",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1136/bmj.315.7109.629",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.3390/jcm9092961",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1016/j.jiph.2021.02.013",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1016/j.jinf.2021.02.008",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1136/rmdopen-2020-001455",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.5005/jp-journals-10071-23599",
"article-title": "A retrospective observational study of hypoxic COVID-19 patients treated with immunomodulatory drugs in a tertiary care hospital",
"author": "Mahale",
"doi-asserted-by": "crossref",
"first-page": "1020",
"journal-title": "Indian J. Crit. Care Med.",
"key": "ref29",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.21203/rs.3.rs-94922/v1",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.1155/2020/8865954",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1136/annrheumdis-2020-217712",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1016/j.jmii.2020.07.024",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.3389/fimmu.2020.583373",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1186/s13054-020-02925-3",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.3389/fimmu.2020.01518",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.3389/fimmu.2020.01021",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.3389/fimmu.2020.01580",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1161/JAHA.115.002128",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1038/s41577-019-0165-0",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1016/j.atherosclerosis.2017.12.027",
"doi-asserted-by": "publisher",
"key": "ref41"
}
],
"reference-count": 41,
"references-count": 41,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2075-1729/11/8/864"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Paleontology",
"Space and Planetary Science",
"General Biochemistry, Genetics and Molecular Biology",
"Ecology, Evolution, Behavior and Systematics"
],
"subtitle": [],
"title": "Repurposing Colchicine in Treating Patients with COVID-19: A Systematic Review and Meta-Analysis",
"type": "journal-article",
"volume": "11"
}
