Colchicine use might be associated with lower mortality in COVID‐19 patients: A meta‐analysis
et al., European Journal of Clinical Investigation, doi:10.1111/eci.13645, Jul 2021
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
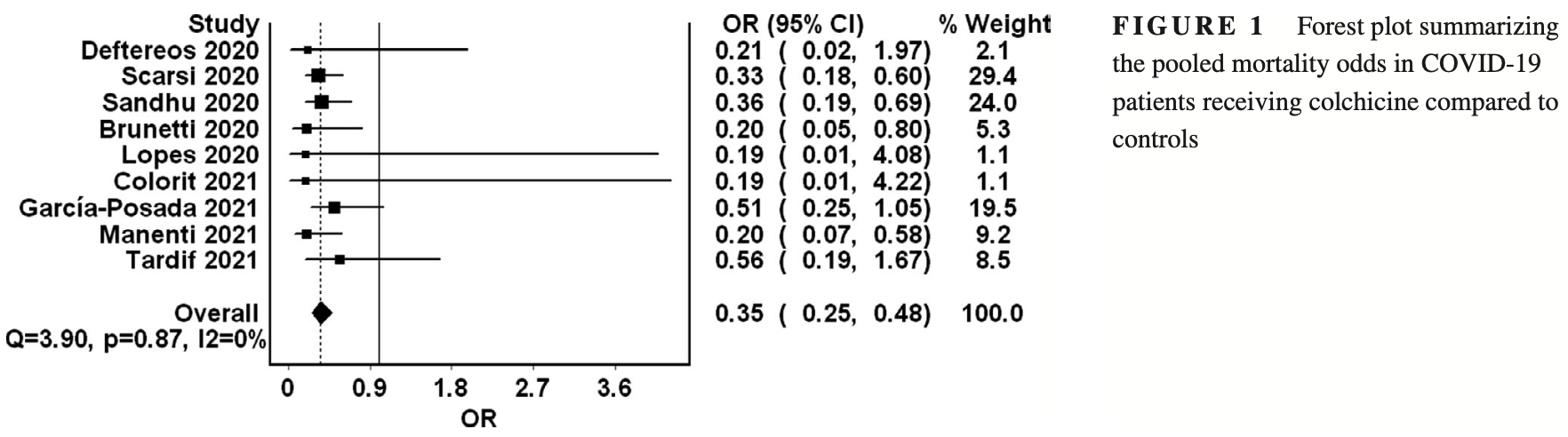
Meta analysis of 9 colchicine studies showing significantly lower mortality with treatment.
10 meta-analyses show significant improvements with colchicine for mortality1-8,
oxygen therapy8,
hospitalization9, and
severity10.
Currently there are 54 colchicine for COVID-19 studies, showing 22% lower mortality [12‑31%], 29% lower ventilation [-15‑56%], 34% lower ICU admission [8‑52%], 17% lower hospitalization [9‑25%], and 9% more cases [-8‑29%].
|
risk of death, 65.0% lower, OR 0.35, p < 0.001, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zein et al., Effect of colchicine on mortality in patients with COVID-19 – A systematic review and meta-analysis, Diabetes & Metabolic Syndrome: Clinical Research & Reviews, doi:10.1016/j.dsx.2022.102395.
2.
Rai et al., The Potential Role of Colchicine in Reducing Mortality and Mechanical Ventilation Rates in COVID-19 Infection: A Meta-analysis, Journal of Advances in Medicine and Medical Research, doi:10.9734/jammr/2022/v34i2031503.
3.
Elshafei et al., Colchicine use might be associated with lower mortality in COVID‐19 patients: A meta‐analysis, European Journal of Clinical Investigation, doi:10.1111/eci.13645.
4.
Lien et al., Repurposing Colchicine in Treating Patients with COVID-19: A Systematic Review and Meta-Analysis, Life, doi:10.3390/life11080864.
5.
Danjuma et al., Does Colchicine Reduce Mortality in Patients with Covid-19 Clinical Syndrome? An Umbrella Review of Published Meta-Analyses, Elsevier BV, doi:10.2139/ssrn.4447127.
6.
Salah et al., Meta-analysis of the Effect of Colchicine on Mortality and Mechanical Ventilation in COVID-19, The American Journal of Cardiology, doi:10.1016/j.amjcard.2021.02.005.
7.
Golpour et al., The effectiveness of Colchicine as an anti-inflammatory drug in the treatment of coronavirus disease 2019: Meta-analysis, International Journal of Immunopathology and Pharmacology, doi:10.1177/20587384211031763.
8.
Elshiwy et al., The role of colchicine in the management of COVID-19: a meta-analysis, BMC Pulmonary Medicine, doi:10.1186/s12890-024-03001-0.
Elshafei et al., 18 Jul 2021, peer-reviewed, 7 authors.
Contact: dr.m.oraiby@hotmail.com.
Colchicine use might be associated with lower mortality in COVID‐19 patients: A meta‐analysis
European Journal of Clinical Investigation, doi:10.1111/eci.13645
Background: Colchicine was recently repurposed for the management of coronavirus disease 2019 (COVID-19). This rapid review and meta-analysis aimed to assess colchicine's impact on mortality outcomes in COVID-19 patients. Materials and Methods: We systematically searched PubMed, EMBASE, Google Scholar since their inception till 25/03/2021 for observational or controlled studies that reported mortality as an outcome. The mortality odd ratios were generated with their corresponding 95% confidence intervals utilizing the random-effects model. Results: Nine studies comprising 5522 patients met our inclusion criteria. Our metaanalysis revealed significantly lower mortality in the colchicine group (OR 0.35, 95% CI 0.25-0.48, I2 0%) compared with controls. A subgroup analysis limited to hospitalized patients (OR 0.35, 95% CI 0.25-0.50, I2 0%) revealed similarly lower mortality in the colchicine group. Conclusions: This meta-analysis suggests a mortality benefit with colchicine when used in the treatment of COVID-19 patients. The majority of included studies were observational; thus, the findings of this review need to be further supported by the results of ongoing trials.
CONFLICT OF INTEREST None declared by all authors.
AUTHOR CONTRIBUTIONS
ETHICAL APPROVAL No ethical approval is necessary as this was a secondary synthesis of published articles.
References
Aimo, Figal, Bayes-Genis, Emdin, Georgiopoulos, Effect of low-dose colchicine in acute and chronic coronary syndromes: a systematic review and meta-analysis, Eur J Clin Invest, doi:10.1111/eci.13464
Brunetti, Diawara, Tsai, Colchicine to Weather the Cytokine Storm in Hospitalized Patients with COVID-19, J Clin Med, doi:10.3390/jcm9092961
Deftereos, Giannopoulos, Vrachatis, Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial, JAMA Netw open, doi:10.1001/jamanetworkopen.2020.13136
Elshafei, Khalil, El-Bardissy, Danjuma, Ahmed et al., The efficacy of colchicine in the management of coronavirus disease 2019: A protocol for systematic review and meta-analysis, Medicine, doi:10.1097/MD.0000000000021911
García-Posada, Vesga, Mestra, Clinical outcomes of patients hospitalized for COVID-19 and evidence-based on the pharmacological management reduce mortality in a region of the Colombian Caribbean, J Infect Public Health, doi:10.1016/j.jiph.2021.02.013
Kimmig, Wu, Gold, IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated With Increased Secondary Infections, Front Med, doi:10.3389/fmed.2020.583897
Lopes, Bonjorno, Giannini, Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial, RMD Open, doi:10.1136/rmdopen-2020-001455
Lopes, Bonjorno, Giannini, Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, doubleblinded, placebo-controlled clinical trial, RMD Open, doi:10.1136/rmdopen-2020-001455
Mahale, Rajhans, Godavarthy, A Retrospective Observational Study of Hypoxic COVID-19 Patients Treated with Immunomodulatory Drugs in a Tertiary Care Hospital, Indian J Crit Care Med, doi:10.5005/jp-journals-10071-23599
Manenti, Maggiore, Fiaccadori, Reduced mortality in COVID-19 patients treated with colchicine: Results from a retrospective, observational study. Cannatà A, PLoS ONE, doi:10.1371/journal.pone.0248276
Mareev, Orlova, Plisyk, Proactive antiinflammatory therapy with colchicine in the treatment of advanced stages of new coronavirus infection. The first results of the COLORIT study, Kardiologiia, doi:10.18087/cardio.2021.2.n1560
Mohamed, Sd, Shunnar, Prevalence of Venous Thromboembolism in Critically Ill COVID-19 Patients: Systematic Review and Meta-Analysis, Front Cardiovasc Med, doi:10.3389/fcvm.2020.598846
Pinzón, Doris Cardona Arango, Betancur, Clinical Outcome of Patients with COVID-19
Sandhu, Tieng, Chilimuri, Franchin, A case control study to evaluate the impact of colchicine on patients admitted to the hospital with moderate to severe covid-19 infection, Can J Infect Dis Med Microbiol, doi:10.1155/2020/8865954
Scarsi, Piantoni, Colombo, Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome, Ann Rheum Dis, doi:10.1136/annrheumdis-2020-217712
Tardif, Bouabdallaoui, Allier, Efficacy of colchicine in non-hospitalized patients with COVID-19, doi:10.1101/2021.01.26.21250494
Wichmann, Sperhake, Lütgehetmann, Autopsy findings and venous thromboembolism in patients with COVID-19
DOI record:
{
"DOI": "10.1111/eci.13645",
"ISSN": [
"0014-2972",
"1365-2362"
],
"URL": "http://dx.doi.org/10.1111/eci.13645",
"alternative-id": [
"10.1111/eci.13645"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-02-10"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2021-06-04"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2021-07-18"
}
],
"author": [
{
"affiliation": [
{
"name": "Clinical Pharmacy Department Hamad Medical Corporation Doha Qatar"
}
],
"family": "Elshafei",
"given": "Mohamed Nabil",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Clinical Pharmacy Department Hamad Medical Corporation Doha Qatar"
}
],
"family": "El‐Bardissy",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Pharmacy Department Hamad Medical Corporation Doha Qatar"
}
],
"family": "Khalil",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Medicine Qatar University Doha Qatar"
},
{
"name": "Department of Medicine Hamad Medical Corporation Doha Qatar"
}
],
"family": "Danjuma",
"given": "Mohammed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine Rochester Regional Health Unity Hospital of Rochester Rochester NY USA"
}
],
"family": "Mubasher",
"given": "Mahmood",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Alpert Medical School Brown University Providence RI USA"
}
],
"family": "Abubeker",
"given": "Ibrahim Y.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4761-8014",
"affiliation": [
{
"name": "Department of Medicine Hamad Medical Corporation Doha Qatar"
}
],
"authenticated-orcid": false,
"family": "Mohamed",
"given": "Mouhand F. H.",
"sequence": "additional"
}
],
"container-title": "European Journal of Clinical Investigation",
"container-title-short": "Eur J Clin Investigation",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2021,
6,
29
]
],
"date-time": "2021-06-29T15:08:13Z",
"timestamp": 1624979293000
},
"deposited": {
"date-parts": [
[
2022,
5,
20
]
],
"date-time": "2022-05-20T14:20:13Z",
"timestamp": 1653056413000
},
"indexed": {
"date-parts": [
[
2023,
2,
18
]
],
"date-time": "2023-02-18T18:52:35Z",
"timestamp": 1676746355519
},
"is-referenced-by-count": 16,
"issue": "9",
"issued": {
"date-parts": [
[
2021,
7,
18
]
]
},
"journal-issue": {
"issue": "9",
"published-print": {
"date-parts": [
[
2021,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
18
]
],
"date-time": "2021-07-18T00:00:00Z",
"timestamp": 1626566400000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
18
]
],
"date-time": "2021-07-18T00:00:00Z",
"timestamp": 1626566400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/eci.13645",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/eci.13645",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/eci.13645",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1111",
"published": {
"date-parts": [
[
2021,
7,
18
]
]
},
"published-online": {
"date-parts": [
[
2021,
7,
18
]
]
},
"published-print": {
"date-parts": [
[
2021,
9
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1111/eci.13464",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_2_1"
},
{
"DOI": "10.3390/jcm9092961",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_3_1"
},
{
"DOI": "10.1136/rmdopen-2020-001455",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_4_1"
},
{
"DOI": "10.3389/fcvm.2020.598846",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_5_1"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_6_1"
},
{
"DOI": "10.1136/annrheumdis-2020-217712",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_7_1"
},
{
"DOI": "10.1097/MD.0000000000021911",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_8_1"
},
{
"article-title": "Efficacy of colchicine in non‐hospitalized patients with COVID‐19",
"author": "Tardif J‐C",
"journal-title": "medRxiv",
"key": "e_1_2_6_9_1",
"year": "2021"
},
{
"DOI": "10.18087/cardio.2021.2.n1560",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_10_1"
},
{
"DOI": "10.1155/2020/8865954",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_11_1"
},
{
"DOI": "10.1371/journal.pone.0248276",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_12_1"
},
{
"DOI": "10.1016/j.jiph.2021.02.013",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_13_1"
},
{
"DOI": "10.21203/rs.3.rs-94922/v1",
"doi-asserted-by": "crossref",
"key": "e_1_2_6_14_1",
"unstructured": "Alejandro PinzónM Medellin Doris Cardona ArangoC Felipe BetancurJ et al.Clinical Outcome of Patients with COVID‐19 Pneumonia Treated with Corticosteroids and Colchicine in Colombia. Published online October 23 2020.https://doi.org/10.21203/rs.3.rs‐94922/v1"
},
{
"DOI": "10.5005/jp-journals-10071-23599",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_15_1"
},
{
"article-title": "IL‐6 Inhibition in Critically Ill COVID‐19 Patients Is Associated With Increased Secondary Infections",
"author": "Kimmig LM",
"journal-title": "Front Med (Lausanne)",
"key": "e_1_2_6_16_1",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.7326/M20-2003",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_17_1"
},
{
"DOI": "10.1136/rmdopen-2020-001455",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_18_1"
}
],
"reference-count": 17,
"references-count": 17,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/eci.13645"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Clinical Biochemistry",
"Biochemistry",
"General Medicine"
],
"subtitle": [],
"title": "Colchicine use might be associated with lower mortality in COVID‐19 patients: A meta‐analysis",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "51"
}
