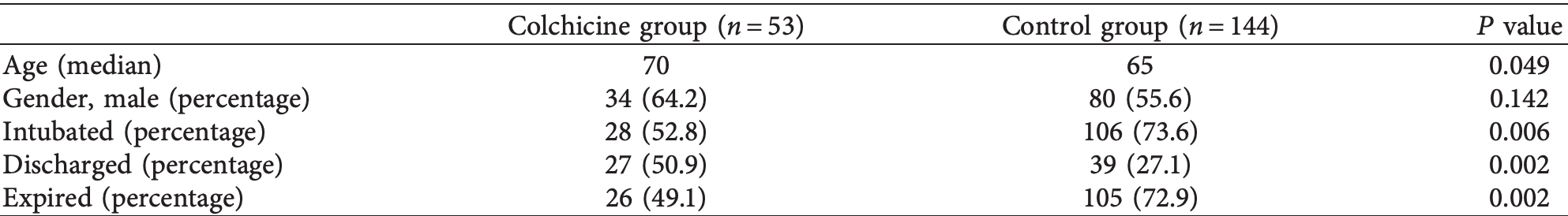
A Case Control Study to Evaluate the Impact of Colchicine on Patients Admitted to the Hospital with Moderate to Severe COVID-19 Infection
et al., Canadian Journal of Infectious Diseases and Medical Microbiology, doi:10.1155/2020/8865954, Oct 2020
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Prospective cohort study of hospitalized patients in the USA, 34 treated with colchicine, showing lower mortality and intubation with treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
|
risk of death, 41.7% lower, RR 0.58, p < 0.001, treatment 16 of 34 (47.1%), control 63 of 78 (80.8%), NNT 3.0.
|
|
risk of mechanical ventilation, 52.9% lower, RR 0.47, p < 0.001, treatment 16 of 34 (47.1%), control 68 of 68 (100.0%), NNT 1.9.
|
|
risk of no hospital discharge, 41.7% lower, RR 0.58, p < 0.001, treatment 16 of 34 (47.1%), control 63 of 78 (80.8%), NNT 3.0.
|
|
hospitalization time, 4.5% lower, relative time 0.95, treatment 34, control 78.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sandhu et al., 27 Oct 2020, prospective, USA, peer-reviewed, 4 authors, dosage 1.2mg days 1-3, 0.6mg days 4-15.
A Case Control Study to Evaluate the Impact of Colchicine on Patients Admitted to the Hospital with Moderate to Severe COVID-19 Infection
doi:10.1155/2020/8865954
Background. Colchicine has been used in conditions such as periodic febrile illness, acute pericarditis, and gouty arthritis, all having a common hyperinflammatory response as seen in moderate to severe forms of coronavirus disease 2019 . is project was carried out during the rapid surge of cases in New York City, and the goal was to assess the efficacy of colchicine in treating patients with COVID-19. Methods. Patients admitted to two distinct pulmonary oriented floors of the BronxCare Hospital Center were compared. Patients on one floor were given colchicine in addition to standard of care, while control patients from another floor received only standard of care. Patients who had at least two separate timepoint measurements for at least two out of four serum inflammatory markers (C-reactive protein (CRP), D-dimer, ferritin, or lactate dehydrogenase (LDH)) were selected for the final comprehensive analysis. Results. An initial analysis performed on all patients, irrespective of the availability of two timepoint inflammatory markers, revealed a lower mortality (49.1% versus 72.9%, P � 0.002), a lower percentage of intubations (52.8% versus 73.6%, P � 0.006), and a higher discharge rate (50.9% versus 27.1%, P � 0.002), in the patients who received colchicine. Patients in the final comprehensive analysis groups (34 in the colchicine group and 78 in the control group) had a similar prevalence of comorbid medical conditions, except for renal failure, which was higher in the control group (65.3% versus 35.2%, P � 0.015). HTN (71.8% versus 52.9%, P � 0.053) and DM (51.3% versus 32.4%, P � 0.064) were also more prevalent in the control group, although the difference was not statistically significant. Patients who received colchicine had a lower mortality than the control group (47.1% versus 80.8%, P � 0.0003), lower rate of intubations (47.1% versus 87.2%, P < 0.0001), and a higher discharge rate (52.9% versus 19.2%, P � 0.0003). Patients in the colchicine group also showed a more significant decrease in inflammatory markers for D-dimer (P � 0.037), CRP (P � 0.014), and ferritin (P � 0.012). Conclusions. Our study demonstrates that colchicine improved outcomes in patients with COVID-19 receiving standard of care therapy. Future randomized, placebo-controlled clinical trials to assess the potential benefit of colchicine in COVID-19 are warranted.
Conflicts of Interest e authors declare that there are no conflicts of interest regarding the publication of this paper.
References
Adapa, Chenna, Balla, COVID-19 pandemic causing acute kidney injury and impact on patients with chronic kidney disease and renal transplantation, Journal of Clinical Medicine Research
Angus, Derde, Al-Beidh, Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19, JAMA
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19-final report, New England Journal of Medicine
Boulware, Pullen, Bangdiwala, A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19, New England Journal of Medicine
Chilimuri, Sun, Alemam, Predictors of mortality in adult population admitted with COVID-19: a retrospective cohort study from New York city, Western Journal of Emergency Medicine: Integrating Emergency Care with Population Health
Deftereos, Giannopoulos, Vrachatis, Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019, JAMA Network Open
Deftereos, Siasos, Giannopoulos, e Greek study in the effects of colchicine in COvid-19 complications prevention (GRECCO-19 study): rationale and study design, Hellenic Journal of Cardiology
Diao, Wang, Tan, Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19), Frontiers in Immunology
Gandolfini, Delsante, Fiaccadori, COVID-19 in kidney transplant recipients, American Journal of Transplantation
Gao, Tian, Yang, Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies, Bioscience Trends
Guaraldi, Meschiari, Cozzi-Lepri, Tocilizumab in patients with severe COVID-19: a retrospective cohort study, e Lancet Rheumatology
Kewan, Covut, Al-Jaghbeer, Rose, Gopalakrishna et al., Tocilizumab for treatment of patients with severe COVID-19: a retrospective cohort study, EClinicalMedicine
Leung, Yao, Hui, Kraus, Colchicineupdate on mechanisms of action and therapeutic uses, Seminars in Arthritis and Rheumatism
Li, Zhao, Wei, Dynamic relationship between D-dimer and COVID-19 severity, British Journal of Haematology
Luo, Liu, Qiu, Liu, Liu et al., Tocilizumab treatment in COVID-19: a single center experience, Journal of Medical Virology
Martínez, Robertson, Barraclough, Colchicine acutely suppresses local cardiac production of inflammatory cytokines in patients with an acute coronary syndrome, Journal of the American Heart Association
Montealegre-Gómez, Garavito, Gómez-López, Rojas-Villarraga, Parra-Medina, Colchicine: a potential therapeutic tool against COVID-19. Experience of 5 patients
Notley, Tinlin, Sawyer, Begbie, Lillicrap, e factor VIII acute phase response requires the participation of NFκB and C/EBP, rombosis and Haemostasis
Shi, Wang, Shao, COVID-19 infection: the perspectives on immune responses, Cell Death & Differentiation
Shukla, Archibald, Shukla, Mehta, Cherabuddi, Chloroquine and hydroxychloroquine in the context of COVID-19, Drugs in Context
Tardif, Kouz, Waters, Efficacy and safety of low-dose colchicine after myocardial infarction, New England Journal of Medicine
Tomazini, Maia, Cavalcanti, Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: e CoDEX randomized clinical trial, JAMA
W.-J. Guan, -Y. Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China, New England Journal of Medicine
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Research
Zhang, Yan, Fan, D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19, Journal of rombosis and Haemostasis
Zhang, Zhao, Zhang, e use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China, Clinical Immunology
DOI record:
{
"DOI": "10.1155/2020/8865954",
"ISSN": [
"1918-1493",
"1712-9532"
],
"URL": "http://dx.doi.org/10.1155/2020/8865954",
"abstract": "<jats:p>Background. Colchicine has been used in conditions such as periodic febrile illness, acute pericarditis, and gouty arthritis, all having a common hyperinflammatory response as seen in moderate to severe forms of coronavirus disease 2019 (COVID-19). This project was carried out during the rapid surge of cases in New York City, and the goal was to assess the efficacy of colchicine in treating patients with COVID-19. Methods. Patients admitted to two distinct pulmonary oriented floors of the BronxCare Hospital Center were compared. Patients on one floor were given colchicine in addition to standard of care, while control patients from another floor received only standard of care. Patients who had at least two separate timepoint measurements for at least two out of four serum inflammatory markers (C-reactive protein (CRP), D-dimer, ferritin, or lactate dehydrogenase (LDH)) were selected for the final comprehensive analysis. Results. An initial analysis performed on all patients, irrespective of the availability of two timepoint inflammatory markers, revealed a lower mortality (49.1% versus 72.9%, <jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M1\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.002</mn>\n </math>\n </jats:inline-formula>), a lower percentage of intubations (52.8% versus 73.6%, <jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M2\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.006</mn>\n </math>\n </jats:inline-formula>), and a higher discharge rate (50.9% versus 27.1%, <jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M3\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.002</mn>\n </math>\n </jats:inline-formula>), in the patients who received colchicine. Patients in the final comprehensive analysis groups (34 in the colchicine group and 78 in the control group) had a similar prevalence of comorbid medical conditions, except for renal failure, which was higher in the control group (65.3% versus 35.2%, <jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M4\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.015</mn>\n </math>\n </jats:inline-formula>). HTN (71.8% versus 52.9%, <jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M5\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.053</mn>\n </math>\n </jats:inline-formula>) and DM (51.3% versus 32.4%, <jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M6\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.064</mn>\n </math>\n </jats:inline-formula>) were also more prevalent in the control group, although the difference was not statistically significant. Patients who received colchicine had a lower mortality than the control group (47.1% versus 80.8%, <jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M7\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.0003</mn>\n </math>\n </jats:inline-formula>), lower rate of intubations (47.1% versus 87.2%, <jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M8\">\n <mi>P</mi>\n <mo><</mo>\n <mn>0.0001</mn>\n </math>\n </jats:inline-formula>), and a higher discharge rate (52.9% versus 19.2%, <jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M9\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.0003</mn>\n </math>\n </jats:inline-formula>). Patients in the colchicine group also showed a more significant decrease in inflammatory markers for D-dimer (<jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M10\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.037</mn>\n </math>\n </jats:inline-formula>), CRP (<jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M11\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.014</mn>\n </math>\n </jats:inline-formula>), and ferritin (<jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M12\">\n <mi>P</mi>\n <mo>=</mo>\n <mn>0.012</mn>\n </math>\n </jats:inline-formula>). Conclusions. Our study demonstrates that colchicine improved outcomes in patients with COVID-19 receiving standard of care therapy. Future randomized, placebo-controlled clinical trials to assess the potential benefit of colchicine in COVID-19 are warranted.</jats:p>",
"alternative-id": [
"8865954",
"8865954"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9253-9868",
"affiliation": [
{
"name": "Department of Internal Medicine, BronxCare Health System, Bronx, NY 10457, USA"
}
],
"authenticated-orcid": true,
"family": "Sandhu",
"given": "Tegveer",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1292-2198",
"affiliation": [
{
"name": "Department of Internal Medicine, BronxCare Health System, Bronx, NY 10457, USA"
}
],
"authenticated-orcid": true,
"family": "Tieng",
"given": "Arlene",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9866-4785",
"affiliation": [
{
"name": "Department of Internal Medicine, BronxCare Health System, Bronx, NY 10457, USA"
}
],
"authenticated-orcid": true,
"family": "Chilimuri",
"given": "Sridhar",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2609-7974",
"affiliation": [
{
"name": "Department of Internal Medicine, BronxCare Health System, Bronx, NY 10457, USA"
}
],
"authenticated-orcid": true,
"family": "Franchin",
"given": "Giovanni",
"sequence": "additional"
}
],
"container-title": "Canadian Journal of Infectious Diseases and Medical Microbiology",
"container-title-short": "Canadian Journal of Infectious Diseases and Medical Microbiology",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
10,
27
]
],
"date-time": "2020-10-27T20:20:06Z",
"timestamp": 1603830006000
},
"deposited": {
"date-parts": [
[
2020,
10,
27
]
],
"date-time": "2020-10-27T20:20:09Z",
"timestamp": 1603830009000
},
"editor": [
{
"affiliation": [],
"family": "Hoepelman",
"given": "Aim",
"sequence": "additional"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
2
]
],
"date-time": "2024-04-02T07:20:15Z",
"timestamp": 1712042415950
},
"is-referenced-by-count": 43,
"issued": {
"date-parts": [
[
2020,
10,
22
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
22
]
],
"date-time": "2020-10-22T00:00:00Z",
"timestamp": 1603324800000
}
}
],
"link": [
{
"URL": "http://downloads.hindawi.com/journals/cjidmm/2020/8865954.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://downloads.hindawi.com/journals/cjidmm/2020/8865954.xml",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://downloads.hindawi.com/journals/cjidmm/2020/8865954.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "98",
"original-title": [],
"page": "1-9",
"prefix": "10.1155",
"published": {
"date-parts": [
[
2020,
10,
22
]
]
},
"published-print": {
"date-parts": [
[
2020,
10,
22
]
]
},
"publisher": "Hindawi Limited",
"reference": [
{
"author": "Centers for Disease Control and Prevention",
"key": "1",
"volume-title": "Coronavirus Disease 2019 (COVID-19) in the U.S.",
"year": "2020"
},
{
"DOI": "10.1016/j.clim.2020.108393",
"doi-asserted-by": "publisher",
"key": "2"
},
{
"DOI": "10.3389/fimmu.2020.00827",
"doi-asserted-by": "publisher",
"key": "3"
},
{
"DOI": "10.1038/s41418-020-0530-3",
"doi-asserted-by": "publisher",
"key": "4"
},
{
"DOI": "10.1016/j.semarthrit.2015.06.013",
"doi-asserted-by": "publisher",
"key": "5"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "6"
},
{
"DOI": "10.5582/bst.2020.01047",
"doi-asserted-by": "publisher",
"key": "7"
},
{
"DOI": "10.1056/NEJMoa2016638",
"doi-asserted-by": "publisher",
"key": "8"
},
{
"DOI": "10.7573/dic.2020-4-5",
"doi-asserted-by": "publisher",
"key": "9"
},
{
"DOI": "10.1002/jmv.25801",
"doi-asserted-by": "publisher",
"key": "10"
},
{
"DOI": "10.1016/j.eclinm.2020.100418",
"doi-asserted-by": "publisher",
"key": "11"
},
{
"DOI": "10.1016/S2665-9913(20)30173-9",
"doi-asserted-by": "publisher",
"key": "12"
},
{
"DOI": "10.1161/jaha.115.002128",
"doi-asserted-by": "publisher",
"key": "13"
},
{
"DOI": "10.1016/j.hjc.2020.03.002",
"doi-asserted-by": "publisher",
"key": "14"
},
{
"DOI": "10.1056/NEJMoa1912388",
"doi-asserted-by": "publisher",
"key": "15"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "16"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"doi-asserted-by": "publisher",
"key": "17"
},
{
"DOI": "10.1016/j.reuma.2020.05.001",
"doi-asserted-by": "publisher",
"key": "18"
},
{
"DOI": "10.1111/ajt.15891",
"doi-asserted-by": "publisher",
"key": "19"
},
{
"DOI": "10.1001/jama.2020.17021",
"doi-asserted-by": "publisher",
"key": "20"
},
{
"DOI": "10.1001/jama.2020.17022",
"doi-asserted-by": "publisher",
"key": "21"
},
{
"DOI": "10.1056/NEJMoa2002032",
"doi-asserted-by": "publisher",
"key": "22"
},
{
"DOI": "10.14740/jocmr4200",
"doi-asserted-by": "publisher",
"key": "23"
},
{
"DOI": "10.5811/westjem.2020.6.47919",
"doi-asserted-by": "publisher",
"key": "24"
},
{
"DOI": "10.1111/jth.14859",
"doi-asserted-by": "publisher",
"key": "25"
},
{
"DOI": "10.1111/bjh.16811",
"doi-asserted-by": "publisher",
"key": "26"
},
{
"DOI": "10.1055/s-0037-1613999",
"doi-asserted-by": "publisher",
"key": "27"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.hindawi.com/journals/cjidmm/2020/8865954/"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "A Case Control Study to Evaluate the Impact of Colchicine on Patients Admitted to the Hospital with Moderate to Severe COVID-19 Infection",
"type": "journal-article",
"volume": "2020"
}
