The effectiveness of Colchicine as an anti-inflammatory drug in the treatment of coronavirus disease 2019: Meta-analysis
et al., International Journal of Immunopathology and Pharmacology, doi:10.1177/20587384211031763, Jul 2021
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
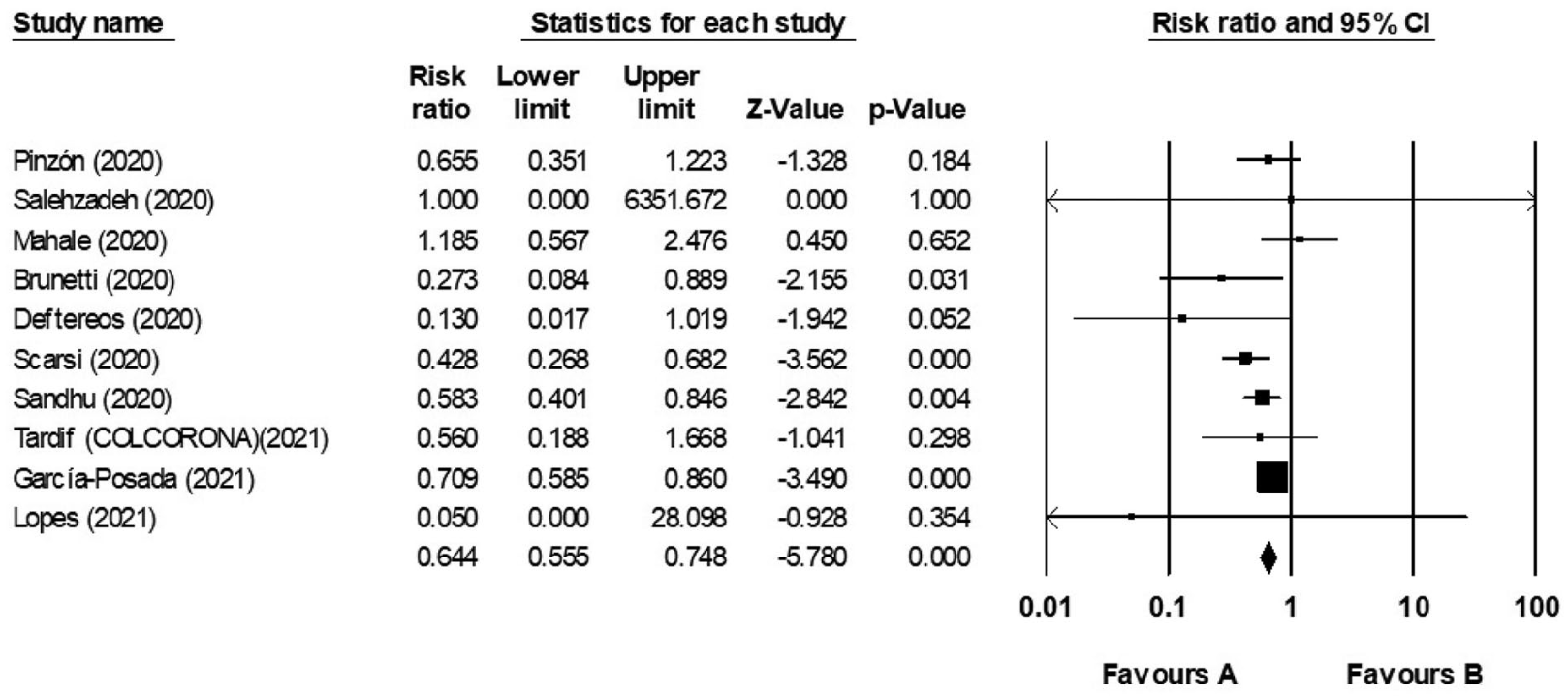
Meta analysis of 10 studies showing significantly lower COVID-19 mortality with colchicine.
10 meta-analyses show significant improvements with colchicine for mortality1-8,
oxygen therapy8,
hospitalization9, and
severity10.
Currently there are 54 colchicine for COVID-19 studies, showing 22% lower mortality [12‑31%], 29% lower ventilation [-15‑56%], 34% lower ICU admission [8‑52%], 17% lower hospitalization [9‑25%], and 9% more cases [-8‑29%].
|
risk of death, 35.6% lower, RR 0.64, p < 0.001.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zein et al., Effect of colchicine on mortality in patients with COVID-19 – A systematic review and meta-analysis, Diabetes & Metabolic Syndrome: Clinical Research & Reviews, doi:10.1016/j.dsx.2022.102395.
2.
Rai et al., The Potential Role of Colchicine in Reducing Mortality and Mechanical Ventilation Rates in COVID-19 Infection: A Meta-analysis, Journal of Advances in Medicine and Medical Research, doi:10.9734/jammr/2022/v34i2031503.
3.
Elshafei et al., Colchicine use might be associated with lower mortality in COVID‐19 patients: A meta‐analysis, European Journal of Clinical Investigation, doi:10.1111/eci.13645.
4.
Lien et al., Repurposing Colchicine in Treating Patients with COVID-19: A Systematic Review and Meta-Analysis, Life, doi:10.3390/life11080864.
5.
Danjuma et al., Does Colchicine Reduce Mortality in Patients with Covid-19 Clinical Syndrome? An Umbrella Review of Published Meta-Analyses, Elsevier BV, doi:10.2139/ssrn.4447127.
6.
Salah et al., Meta-analysis of the Effect of Colchicine on Mortality and Mechanical Ventilation in COVID-19, The American Journal of Cardiology, doi:10.1016/j.amjcard.2021.02.005.
7.
Golpour et al., The effectiveness of Colchicine as an anti-inflammatory drug in the treatment of coronavirus disease 2019: Meta-analysis, International Journal of Immunopathology and Pharmacology, doi:10.1177/20587384211031763.
8.
Elshiwy et al., The role of colchicine in the management of COVID-19: a meta-analysis, BMC Pulmonary Medicine, doi:10.1186/s12890-024-03001-0.
Golpour et al., 10 Jul 2021, peer-reviewed, 7 authors.
Contact: rafiei1710@gmail.com.
The effectiveness of Colchicine as an anti-inflammatory drug in the treatment of coronavirus disease 2019: Meta-analysis
International Journal of Immunopathology and Pharmacology, doi:10.1177/20587384211031763
A recently discovered coronavirus, SARS-CoV-2, caused a global respiratory disease pandemic called COVID-19. Many studies have shown the excessive activation of the innate immune response that leads to the adverse outcomes of COVID-19, and anti-inflammatory drugs are very useful in the treatment and management of this infection. The activities of Colchicine, one of the anti-inflammatory drugs, target several pathways related to excessive inflammation of COVID-19. This study aimed to evaluate the efficacy of Colchicine in the treatment of COVID-19 using a meta-analysis approach. Scopus, Pubmed, Google scholars, Web of Science, and Science direct were used to search all the randomized controlled trials, case-control, and cross-sectional studies that have evaluated the efficacy of Colchicine as a treatment for COVID-19 (up to 28 May 2021). The overall effect of Colchicine versus the control group was determined using a random-effects model meta-analysis where we compared changes (i.e. mean differences-Colchicine group vs Control group) between the two conditions in test scores indicative of hospitalization time (day) and mortality rate. The results illustrated Colchicine therapy is associated with a decreased mortality rate in COVID-19 patients and associated with a decrease in hospitalization time (day) in COVID-19 patients. Present preliminary data shows that Colchicine has a beneficial effect on coronavirus disease care in 2019. Therefore, Colchicine can be a good suggestion in the management of COVID-19.
Declaration of conflicting interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
Angelidis, Kotsialou, Kossyvakis, Colchicine pharmacokinetics and mechanism of action, Current Pharmaceutical Design
Brunetti, Diawara, Tsai, Colchicine to weather the cytokine storm in hospitalized patients with COVID-19, Journal of Clinical Medicine
Clark, Wells, Huët, Assessing the quality of randomized trials: reliability of the Jadad scale, Controlled Clinical Trials
Cullen, Palmer-Cooper, Hardwick, Influence of methodological and patient factors on serum NMDAR IgG antibody detection in psychotic disorders: a meta-analysis of cross-sectional and case-control studies, The Lancet Psychiatry
Dalili, Dalili, Kashefizadeh, Adding colchicine to the antiretroviral medicationlopinavir/ritonavir (Kaletra) in hospitalized patients with non-severe Covid-19 pneumonia: A structured summary of a study protocol for a randomized controlled trial, Trials
Deftereos, Giannopoulos, Vrachatis, Colchicine as a potent anti-inflammatory treatment in COVID-19: Can we teach an old dog new tricks?, European Heart Journal Cardiovascular Pharmacotherapy
Deftereos, Giannopoulos, Vrachatis, Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: The GRECCO-19 randomized clinical trial, JAMA Network Open
Deftereos, Siasos, Giannopoulos, The Greek study in the effects of colchicine in COVID-19 complications prevention (GRECCO-19 study): Rationale and study design, Hellenic Journal of Cardiology
Fara, Mitrev, Rosalia, Cytokine storm and COVID-19: A chronicle of pro-inflammatory cytokines, Open Biology
Freeman, Swartz, Targeting the NLRP3 inflammasome in severe COVID-19, Frontiers in Immunology
Gandolfini, Delsante, Fiaccadori, COVID-19 in kidney transplant recipients, American Journal of Transplantation
García-Posada, Vesga, Mestra, Clinical outcomes of patients hospitalized for COVID-19 and evidence-based on the pharmacological management reduce mortality in a region of the Colombian Caribbean, Journal of Infection and Public Health
Leung, Hui, Kraus, Colchicine-Update on Mechanisms of Action and Therapeutic Uses, Seminars in Arthritis and Rheumatism
Lopes, Bonjorno, Giannini, Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebocontrolled clinical trial, RMD Open
Lopes, Bonjorno, Giannini, Beneficial effects of colchicine for moderate to severe COVID-19: an interim analysis of a randomized, double-blinded, placebo controlled clinical trial, MedRxiv, doi:10.1101/2020.08.06.20169573
Mahale, Rajhans, Godavarthy, A retrospective observational study of hypoxic COVID-19 patients treated with immunomodulatory drugs in a tertiary care hospital, Indian Journal of Critical Care Medicine
Mansouri, Marjani, Tabarsi, Successful treatment of Covid-19 associated cytokine release syndrome with colchicine. A case report and review of literature, Immunological Investigations. Epub, doi:10.1080/08820139.2020.1789655
Montealegre-Gómez, Garavito, Gómez-López, Colchicina: una herramienta terapéutica potencial frente a COVID-19. Experiencia en 5 pacientes. Reumatología Clínica, doi:10.1016/j.reuma.2020.05.001
Monti, Montecucco, Candidate rheumatologic treatments for COVID-19. Response to: 'COVID-19 infection in a patient with FMF: Does colchicine have a protective effect?' by Kobak, Annals of the Rheumatic Diseases
Moore, Ch, Cytokine release syndrome in severe COVID-19, Science
Nidorf, Fiolet, Mosterd, Colchicine in patients with chronic coronary disease, New England Journal of Medicine
Pinzón, Arango, Betancur, Clinical outcome of patients with COVID-19 pneumonia treated with corticosteroids and colchicine in Colombia
Salehzadeh, Pourfarzi, Ataei, The impact of colchicine on the COVID-19 patients: A clinical trial study
Salehzadeh, Pourfarzi, Ataei, The impact of colchicine on the COVID-19 patients; a clinical trial study
Sandhu, Tieng, Chilimuri, A case control study to evaluate the impact of colchicine on patients admitted to the hospital with moderate to severe COVID-19 infection, The Canadian Journal of Infectious Diseases & Medical Microbiology
Scarsi, Piantoni, Colombo, Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome, Annals of the Rheumatic Diseases
Slobodnick, Shah, Krasnokutsky, Update on colchicine, Rheumatology
Tahaghoghi-Hajghorbani, Zafari, Masoumi, The role of dysregulated immune responses in COVID-19 pathogenesis, Virus Research
Tardif, Bouabdallaoui, Allier, Efficacy of colchicine in non-hospitalized patients with COVID-19, doi:10.1101/2021.01.26.21250494
Thompson, Nidorf, Colchicine: An affordable anti-inflammatory agent for atherosclerosis, Current Opinion in Lipidology
Vitiello, Ferrara, Ferrara, Colchicine and SARS-CoV-2: Management of the hyperinflammatory state, Respiratory Medicine
Vitiello, Ferrara, Pelliccia, Cytokine storm and colchicine potential role in fighting SARS-CoV-2 pneumonia, Italian Journal of Medicine
DOI record:
{
"DOI": "10.1177/20587384211031763",
"ISSN": [
"2058-7384",
"2058-7384"
],
"URL": "http://dx.doi.org/10.1177/20587384211031763",
"abstract": "<jats:p> A recently discovered coronavirus, SARS-CoV-2, caused a global respiratory disease pandemic called COVID-19. Many studies have shown the excessive activation of the innate immune response that leads to the adverse outcomes of COVID-19, and anti-inflammatory drugs are very useful in the treatment and management of this infection. The activities of Colchicine, one of the anti-inflammatory drugs, target several pathways related to excessive inflammation of COVID-19. This study aimed to evaluate the efficacy of Colchicine in the treatment of COVID-19 using a meta-analysis approach. Scopus, Pubmed, Google scholars, Web of Science, and Science direct were used to search all the randomized controlled trials, case-control, and cross-sectional studies that have evaluated the efficacy of Colchicine as a treatment for COVID-19 (up to 28 May 2021). The overall effect of Colchicine versus the control group was determined using a random-effects model meta-analysis where we compared changes (i.e. mean differences—Colchicine group vs Control group) between the two conditions in test scores indicative of hospitalization time (day) and mortality rate. The results illustrated Colchicine therapy is associated with a decreased mortality rate in COVID-19 patients and associated with a decrease in hospitalization time (day) in COVID-19 patients. Present preliminary data shows that Colchicine has a beneficial effect on coronavirus disease care in 2019. Therefore, Colchicine can be a good suggestion in the management of COVID-19. </jats:p>",
"alternative-id": [
"10.1177/20587384211031763"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-1406-9028",
"affiliation": [
{
"name": "Molecular and Cell Biology Research Center, Student Research Committee, Faculty of Medicine, Mazandaran University of Medical Science, Sari, Iran"
}
],
"authenticated-orcid": false,
"family": "Golpour",
"given": "Monireh",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Molecular and Cell Biology Research Center, Hemoglobinopathy Research Institute, Mazandaran University of Medical Sciences, Sari, Iran"
}
],
"family": "Mousavi",
"given": "Tahoora",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Immunology, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran"
}
],
"family": "Alimohammadi",
"given": "Mina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Immunology, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran"
}
],
"family": "Mosayebian",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Physiology and Pharmacology, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran"
}
],
"family": "Shiran",
"given": "Mohammadreza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gastrointestinal Cancer Research Center, Non-Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran"
}
],
"family": "Alizadeh Navaei",
"given": "Reza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Immunology, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran"
}
],
"family": "Rafiei",
"given": "Alireza",
"sequence": "additional"
}
],
"container-title": "International Journal of Immunopathology and Pharmacology",
"container-title-short": "Int J Immunopathol Pharmacol",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2021,
7,
10
]
],
"date-time": "2021-07-10T10:39:41Z",
"timestamp": 1625913581000
},
"deposited": {
"date-parts": [
[
2021,
7,
10
]
],
"date-time": "2021-07-10T10:39:53Z",
"timestamp": 1625913593000
},
"indexed": {
"date-parts": [
[
2023,
5,
15
]
],
"date-time": "2023-05-15T09:12:50Z",
"timestamp": 1684141970412
},
"is-referenced-by-count": 20,
"issued": {
"date-parts": [
[
2021,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
1
]
],
"date-time": "2021-01-01T00:00:00Z",
"timestamp": 1609459200000
}
}
],
"link": [
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/20587384211031763",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/full-xml/10.1177/20587384211031763",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/20587384211031763",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"page": "205873842110317",
"prefix": "10.1177",
"published": {
"date-parts": [
[
2021,
1
]
]
},
"published-online": {
"date-parts": [
[
2021,
7,
10
]
]
},
"published-print": {
"date-parts": [
[
2021,
1
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1016/j.hjc.2020.03.002",
"doi-asserted-by": "publisher",
"key": "bibr1-20587384211031763"
},
{
"DOI": "10.1016/j.virusres.2020.198197",
"doi-asserted-by": "publisher",
"key": "bibr2-20587384211031763"
},
{
"DOI": "10.1098/rsob.200160",
"doi-asserted-by": "publisher",
"key": "bibr3-20587384211031763"
},
{
"DOI": "10.3389/fimmu.2020.01518",
"doi-asserted-by": "publisher",
"key": "bibr4-20587384211031763"
},
{
"DOI": "10.1126/science.abb8925",
"doi-asserted-by": "publisher",
"key": "bibr5-20587384211031763"
},
{
"DOI": "10.1093/ehjcvp/pvaa033",
"doi-asserted-by": "publisher",
"key": "bibr6-20587384211031763"
},
{
"DOI": "10.1056/NEJMoa2021372",
"doi-asserted-by": "publisher",
"key": "bibr7-20587384211031763"
},
{
"DOI": "10.1093/rheumatology/kex453",
"doi-asserted-by": "publisher",
"key": "bibr8-20587384211031763"
},
{
"DOI": "10.2174/1381612824666180123110042",
"doi-asserted-by": "publisher",
"key": "bibr9-20587384211031763"
},
{
"DOI": "10.1097/MOL.0000000000000552",
"doi-asserted-by": "publisher",
"key": "bibr10-20587384211031763"
},
{
"author": "Leung YY",
"key": "bibr11-20587384211031763",
"volume-title": "Colchicine—Update on Mechanisms of Action and Therapeutic Uses. Seminars in Arthritis and Rheumatism",
"year": "2015"
},
{
"DOI": "10.1186/s13063-020-04455-3",
"doi-asserted-by": "publisher",
"key": "bibr12-20587384211031763"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"doi-asserted-by": "publisher",
"key": "bibr13-20587384211031763"
},
{
"DOI": "10.1080/08820139.2020.1789655.",
"doi-asserted-by": "publisher",
"key": "bibr14-20587384211031763"
},
{
"author": "Sandhu T",
"journal-title": "The Canadian Journal of Infectious Diseases & Medical Microbiology",
"key": "bibr15-20587384211031763",
"volume": "8865954",
"year": "2020"
},
{
"DOI": "10.1136/annrheumdis-2020-217712",
"doi-asserted-by": "publisher",
"key": "bibr16-20587384211031763"
},
{
"DOI": "10.1016/S0197-2456(99)00026-4",
"doi-asserted-by": "publisher",
"key": "bibr17-20587384211031763"
},
{
"DOI": "10.1016/S2215-0366(20)30432-6",
"doi-asserted-by": "publisher",
"key": "bibr18-20587384211031763"
},
{
"DOI": "10.1136/rmdopen-2020-001455",
"doi-asserted-by": "publisher",
"key": "bibr21-20587384211031763"
},
{
"DOI": "10.3390/jcm9092961",
"doi-asserted-by": "publisher",
"key": "bibr22-20587384211031763"
},
{
"author": "Tardif J-C",
"journal-title": "Medrxiv",
"key": "bibr23-20587384211031763",
"year": "2021"
},
{
"DOI": "10.5005/jp-journals-10071-23599",
"doi-asserted-by": "publisher",
"key": "bibr24-20587384211031763"
},
{
"DOI": "10.1016/j.jiph.2021.02.013",
"doi-asserted-by": "publisher",
"key": "bibr25-20587384211031763"
},
{
"DOI": "10.1016/j.rmed.2021.106322",
"doi-asserted-by": "publisher",
"key": "bibr26-20587384211031763"
},
{
"author": "Vitiello A",
"first-page": "88",
"issue": "2",
"journal-title": "Italian Journal of Medicine",
"key": "bibr27-20587384211031763",
"volume": "14",
"year": "2020"
},
{
"author": "Monti S",
"first-page": "39",
"journal-title": "Annals of the Rheumatic Diseases",
"key": "bibr28-20587384211031763",
"volume": "80",
"year": "2020"
},
{
"DOI": "10.1111/ajt.15891",
"doi-asserted-by": "publisher",
"key": "bibr29-20587384211031763"
},
{
"DOI": "10.1016/j.reuma.2020.05.001.",
"doi-asserted-by": "publisher",
"key": "bibr30-20587384211031763"
},
{
"author": "Lopes MIF",
"journal-title": "MedRxiv",
"key": "bibr31-20587384211031763",
"year": "2020"
},
{
"author": "Salehzadeh F",
"journal-title": "BMC Infectious Diseases",
"key": "bibr32-20587384211031763",
"year": "2020"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "http://journals.sagepub.com/doi/10.1177/20587384211031763"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology",
"Immunology",
"Immunology and Allergy",
"Pharmacology",
"Immunology",
"Immunology and Allergy"
],
"subtitle": [],
"title": "The effectiveness of Colchicine as an anti-inflammatory drug in the treatment of coronavirus disease 2019: Meta-analysis",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy",
"volume": "35"
}
