The effect of colchicine on mortality outcome and duration of hospital stay in patients with COVID‐19: A meta‐analysis of randomized trials
et al., Immunity, Inflammation and Disease, doi:10.1002/iid3.562, Dec 2021
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Meta-analysis of 10 randomized controlled trials with 17,976 COVID-19 patients showing significantly shorter hospitalization with colchicine treatment. Mortality was lower but without statistical significance.
10 meta-analyses show significant improvements with colchicine for mortality1-8,
oxygen therapy8,
hospitalization9, and
severity10.
Currently there are 54 colchicine for COVID-19 studies, showing 22% lower mortality [12‑31%], 29% lower ventilation [-15‑56%], 34% lower ICU admission [8‑52%], 17% lower hospitalization [9‑25%], and 9% more cases [-8‑29%].
1.
Zein et al., Effect of colchicine on mortality in patients with COVID-19 – A systematic review and meta-analysis, Diabetes & Metabolic Syndrome: Clinical Research & Reviews, doi:10.1016/j.dsx.2022.102395.
2.
Rai et al., The Potential Role of Colchicine in Reducing Mortality and Mechanical Ventilation Rates in COVID-19 Infection: A Meta-analysis, Journal of Advances in Medicine and Medical Research, doi:10.9734/jammr/2022/v34i2031503.
3.
Elshafei et al., Colchicine use might be associated with lower mortality in COVID‐19 patients: A meta‐analysis, European Journal of Clinical Investigation, doi:10.1111/eci.13645.
4.
Lien et al., Repurposing Colchicine in Treating Patients with COVID-19: A Systematic Review and Meta-Analysis, Life, doi:10.3390/life11080864.
5.
Danjuma et al., Does Colchicine Reduce Mortality in Patients with Covid-19 Clinical Syndrome? An Umbrella Review of Published Meta-Analyses, Elsevier BV, doi:10.2139/ssrn.4447127.
6.
Salah et al., Meta-analysis of the Effect of Colchicine on Mortality and Mechanical Ventilation in COVID-19, The American Journal of Cardiology, doi:10.1016/j.amjcard.2021.02.005.
7.
Golpour et al., The effectiveness of Colchicine as an anti-inflammatory drug in the treatment of coronavirus disease 2019: Meta-analysis, International Journal of Immunopathology and Pharmacology, doi:10.1177/20587384211031763.
8.
Elshiwy et al., The role of colchicine in the management of COVID-19: a meta-analysis, BMC Pulmonary Medicine, doi:10.1186/s12890-024-03001-0.
Kow et al., 30 Dec 2021, peer-reviewed, 6 authors.
Contact: long.ming@ubd.edu.bn, pohhui.goh@ubd.edu.bn.
The effect of colchicine on mortality outcome and duration of hospital stay in patients with COVID‐19: A meta‐analysis of randomized trials
Immunity, Inflammation and Disease, doi:10.1002/iid3.562
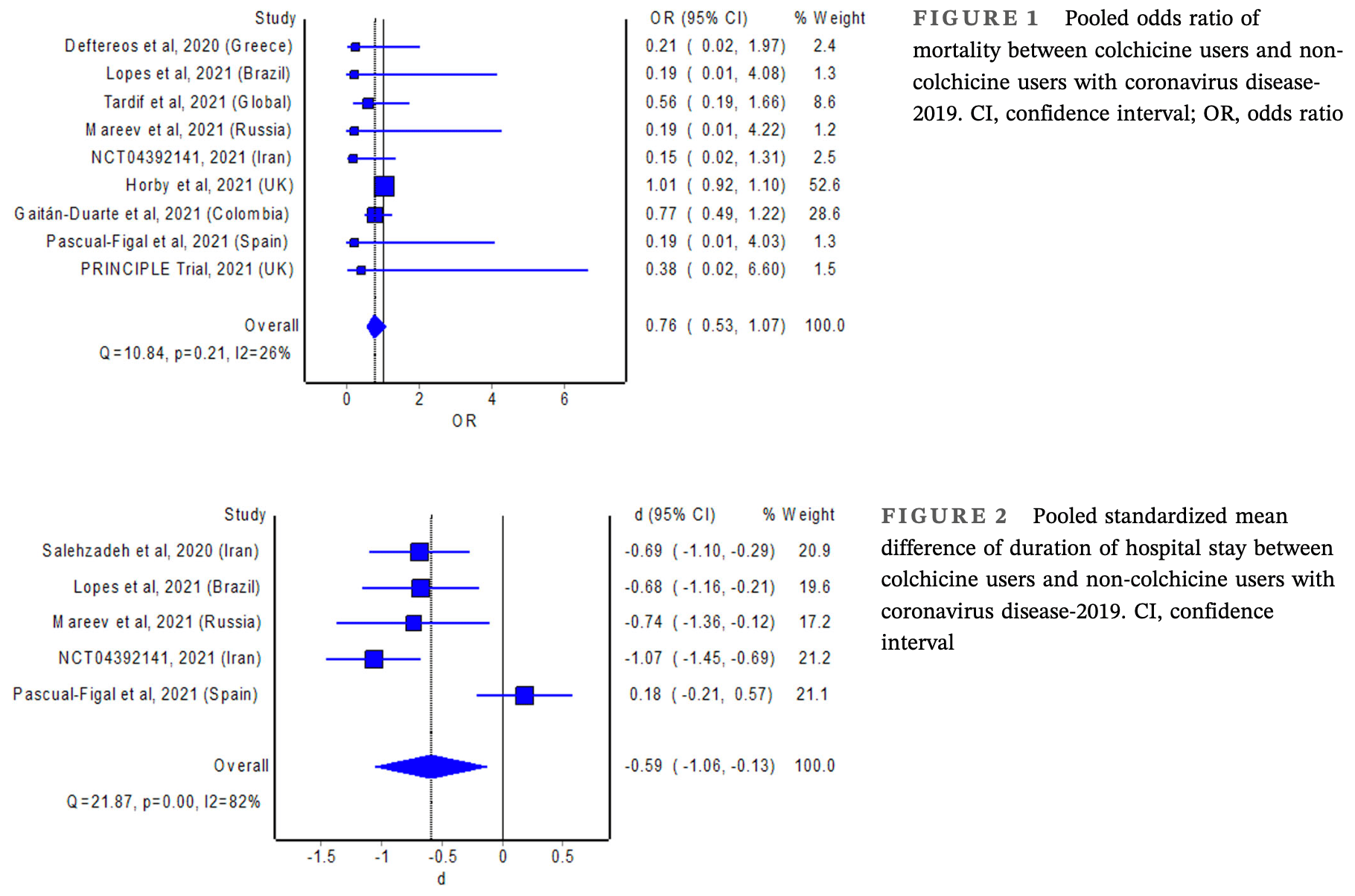
Background: Overactivation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome can lead to severe illness in patients with coronavirus disease-2019 (COVID-19). The NLRP3 inhibitor, colchicine, therefore, appears to be promising for the treatment of COVID-19. Aims: We aimed to perform a meta-analysis of randomized trials investigating the effect of colchicine in patients with COVID-19. Materials & Methods: We systematically searched electronic databases and clinical trial registries (up to October 17, 2021) for eligible studies. The outcomes of interest were all-cause mortality and duration of hospital stay. Metaanalysis with the random-effects model was used to estimate the pooled odds ratio (OR) of mortality and 95% confidence interval (CI). The pooled standardized mean difference of duration of hospital stay with 95% CI between colchicine users and non-colchicine users was estimated using Cohen's d index. Results: The meta-analyses revealed no significant difference in the odds of mortality (pooled OR = 0.76; 95% CI: 0.53-1.07), but a significant reduction in the duration of hospital stay with the use of colchicine (pooled standardized mean difference = -0.59; 95% CI: -1.06 to -0.13).
Discussion and Conclusion: The ability of colchicine to reduce the length of stay in hospitalized patients with COVID-19 is consistent with its potential to prevent clinical deterioration via inhibition of NLRP3 inflammasome. Nevertheless, such beneficial effects of colchicine did not translate into mortality benefits in patients with COVID-19.
CONFLICT OF INTERESTS The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
References
Bertocchi, Foglietta, Collotta, The hidden role of NLRP3 inflammasome in obesity-related COVID-19 exacerbations: lessons for drug repurposing, Br J Pharmacol
Corona, Pizzocaro, Vena, Diabetes is most important cause for mortality in COVID-19 hospitalized patients: systematic review and meta-analysis, Rev Endocr Metab Disord
Da, Ae, Moral-Escudero, Colchicine in recently hospitalized patients with COVID-19: a randomized controlled trial (COL-COVID), Int J Gen Med
Deftereos, Giannopoulos, Vrachatis, Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial, JAMA Netw Open
Demidowich, Levine, Apps, Colchicine's effects on metabolic and inflammatory molecules in adults with obesity and metabolic syndrome: results from a pilot randomized controlled trial, Int J Obes
Dorward, Yu, Hayward, Colchicine for COVID-19 in adults in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial
Fordham, Kirwan, Cason, Currey, Prolonged reduction in polymorphonuclear adhesion following oral colchicine, Ann Rheum Dis
Gaitán-Duarte, Rincon-Rodriguez, Gonzalez, Effectiveness of rosuvastatin plus colchicine, emtricitabine/tenofovir and a combination of them in hospitalized patients with SARS Covid-19
Lambadiari, Kousathana, Raptis, Katogiannis, Kokkinos et al., Pre-existing cytokine and NLRP3 inflammasome activation and increased vascular permeability in diabetes: a possible fatal link with worst COVID-19 infection outcomes?, Front Immunol
Lopes, Bonjorno, Giannini, Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial, RMD Open
Luo, Wan, Liu, Tong, Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range, Stat Methods Med Res
López-Reyes, Martinez-Armenta, Espinosa-Velázquez, NLRP3 inflammasome: the stormy link between obesity and COVID-19, Front Immunol
Mareev, Orlova, Plisyk, Proactive antiinflammatory therapy with colchicine in the treatment of advanced stages of new coronavirus infection. The first results of the COLORIT study, Kardiologiia
Martinon, Pétrilli, Mayor, Tardivel, Tschopp, Goutassociated uric acid crystals activate the NALP3 inflammasome, Nature
Rodrigues, De Sá, Ishimoto, Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients, J Exp Med
Salehzadeh, Pourfarzi, Ataei, The impact of colchicine on the COVID-19 patients: a clinical trial study
Shi, Luo, Detecting the skewness of data from the sample size and the five-number summary
Sterne, Savović, Page, RoB 2: a revised tool for assessing risk of bias in randomised trials, BMJ
Tardif, Bouabdallaoui, Allier, Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebocontrolled, multicentre trial, The Lancet Respiratory Medicine, doi:10.1016/s2213-2600(21)00222-8
Wan, Wang, Liu, Tong, Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range, BMC Med Res Methodol
DOI record:
{
"DOI": "10.1002/iid3.562",
"ISSN": [
"2050-4527",
"2050-4527"
],
"URL": "http://dx.doi.org/10.1002/iid3.562",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>Overactivation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome can lead to severe illness in patients with coronavirus disease‐2019 (COVID‐19). The NLRP3 inhibitor, colchicine, therefore, appears to be promising for the treatment of COVID‐19.</jats:p></jats:sec><jats:sec><jats:title>Aims</jats:title><jats:p>We aimed to perform a meta‐analysis of randomized trials investigating the effect of colchicine in patients with COVID‐19.</jats:p></jats:sec><jats:sec><jats:title>Materials & Methods</jats:title><jats:p>We systematically searched electronic databases and clinical trial registries (up to October 17, 2021) for eligible studies. The outcomes of interest were all‐cause mortality and duration of hospital stay. Meta‐analysis with the random‐effects model was used to estimate the pooled odds ratio (OR) of mortality and 95% confidence interval (CI). The pooled standardized mean difference of duration of hospital stay with 95% CI between colchicine users and non‐colchicine users was estimated using Cohen's d index.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>The meta‐analyses revealed no significant difference in the odds of mortality (pooled OR = 0.76; 95% CI: 0.53–1.07), but a significant reduction in the duration of hospital stay with the use of colchicine (pooled standardized mean difference = −0.59; 95% CI: −1.06 to −0.13).</jats:p></jats:sec><jats:sec><jats:title>Discussion and Conclusion</jats:title><jats:p>The ability of colchicine to reduce the length of stay in hospitalized patients with COVID‐19 is consistent with its potential to prevent clinical deterioration via inhibition of NLRP3 inflammasome. Nevertheless, such beneficial effects of colchicine did not translate into mortality benefits in patients with COVID‐19.</jats:p></jats:sec>",
"alternative-id": [
"10.1002/iid3.562"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-08-11"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2021-10-29"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2021-12-30"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8186-2926",
"affiliation": [
{
"name": "School of Postgraduate Studies International Medical University Kuala Lumpur Malaysia"
},
{
"name": "School of Pharmacy Monash University Malaysia Subang Jaya Selangor Malaysia"
}
],
"authenticated-orcid": false,
"family": "Kow",
"given": "Chia Siang",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Novel Bacteria and Drug Discovery Research Group (NBDD), Microbiome and Bioresource Research Strength (MBRS), Jeffrey Cheah School of Medicine and Health Sciences Monash University Malaysia Subang Jaya Selangor Malaysia"
}
],
"family": "Lee",
"given": "Learn‐Han",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5390-7026",
"affiliation": [
{
"name": "School of Pharmacy Monash University Malaysia Subang Jaya Selangor Malaysia"
}
],
"authenticated-orcid": false,
"family": "Ramachandram",
"given": "Dinesh Sangarran",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4058-2215",
"affiliation": [
{
"name": "Department of Pharmacy University of Huddersfield Huddersfield UK"
},
{
"name": "School of Biomedical Sciences and Pharmacy University of Newcastle Callaghan Australia"
}
],
"authenticated-orcid": false,
"family": "Hasan",
"given": "Syed Shahzad",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6971-1383",
"affiliation": [
{
"name": "PAP Rashidah Sa'adatul Bolkiah Institute of Health Sciences Universiti Brunei Darussalam Gadong Brunei Darussalam"
}
],
"authenticated-orcid": false,
"family": "Ming",
"given": "Long Chiau",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0480-399X",
"affiliation": [
{
"name": "PAP Rashidah Sa'adatul Bolkiah Institute of Health Sciences Universiti Brunei Darussalam Gadong Brunei Darussalam"
}
],
"authenticated-orcid": false,
"family": "Goh",
"given": "Hui Poh",
"sequence": "additional"
}
],
"container-title": "Immunity, Inflammation and Disease",
"container-title-short": "Immunity Inflam &amp; Disease",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2021,
12,
31
]
],
"date-time": "2021-12-31T06:53:53Z",
"timestamp": 1640933633000
},
"deposited": {
"date-parts": [
[
2023,
8,
28
]
],
"date-time": "2023-08-28T08:40:36Z",
"timestamp": 1693212036000
},
"indexed": {
"date-parts": [
[
2024,
1,
18
]
],
"date-time": "2024-01-18T02:21:22Z",
"timestamp": 1705544482264
},
"is-referenced-by-count": 14,
"issue": "2",
"issued": {
"date-parts": [
[
2021,
12,
30
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2022,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
30
]
],
"date-time": "2021-12-30T00:00:00Z",
"timestamp": 1640822400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/iid3.562",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/iid3.562",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/iid3.562",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "255-264",
"prefix": "10.1002",
"published": {
"date-parts": [
[
2021,
12,
30
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
30
]
]
},
"published-print": {
"date-parts": [
[
2022,
2
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1007/s11154-021-09630-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_2_1"
},
{
"DOI": "10.1111/bph.15229",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_3_1"
},
{
"DOI": "10.1084/jem.20201707",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_4_1"
},
{
"DOI": "10.1038/nature04516",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_5_1"
},
{
"DOI": "10.1136/ard.40.6.605",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_6_1"
},
{
"DOI": "10.1038/s41366-020-0598-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_7_1"
},
{
"DOI": "10.1136/bmj.l4898",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_8_1"
},
{
"article-title": "Detecting the skewness of data from the sample size and the five‐number summary",
"author": "Shi J",
"journal-title": "arXiv",
"key": "e_1_2_8_9_1",
"year": "2020"
},
{
"DOI": "10.1177/0962280216669183",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_10_1"
},
{
"DOI": "10.1186/1471-2288-14-135",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_11_1"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_12_1"
},
{
"article-title": "The impact of colchicine on the COVID‐19 patients: a clinical trial study",
"author": "Salehzadeh F",
"journal-title": "Research Square",
"key": "e_1_2_8_13_1",
"year": "2020"
},
{
"DOI": "10.1136/rmdopen-2020-001455",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_14_1"
},
{
"DOI": "10.1016/S2213-2600(21)00222-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_15_1"
},
{
"DOI": "10.18087/cardio.2021.2.n1560",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_16_1"
},
{
"key": "e_1_2_8_17_1",
"unstructured": "Colchicine plus phenolic monoterpenes to treat COVID‐19.ClinicalTrials.govidentifier: NCT04392141. April 20 2021. Accessed July 11 2021.https://www.clinicaltrials.gov/ct2/show/NCT04392141"
},
{
"article-title": "Effectiveness of rosuvastatin plus colchicine, emtricitabine/tenofovir and a combination of them in hospitalized patients with SARS Covid‐19",
"author": "Gaitán‐Duarte HG",
"journal-title": "medRxiv",
"key": "e_1_2_8_18_1",
"year": "2021"
},
{
"key": "e_1_2_8_19_1",
"unstructured": "The Lancet Respiratory Medicine 2021 Colchicine in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised controlled open‐label platform trial"
},
{
"DOI": "10.2147/IJGM.S329810",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_20_1"
},
{
"article-title": "Colchicine for COVID‐19 in adults in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial",
"author": "Dorward J",
"journal-title": "medRxiv",
"key": "e_1_2_8_21_1",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2020.570251",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_22_1"
},
{
"DOI": "10.3389/fimmu.2020.557235",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_23_1"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/iid3.562"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Immunology",
"Immunology and Allergy"
],
"subtitle": [],
"title": "The effect of colchicine on mortality outcome and duration of hospital stay in patients with COVID‐19: A meta‐analysis of randomized trials",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "10"
}
