The Potential Role of Colchicine in Reducing Mortality and Mechanical Ventilation Rates in COVID-19 Infection: A Meta-analysis
et al., Journal of Advances in Medicine and Medical Research, doi:10.9734/jammr/2022/v34i2031503, Jul 2022
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
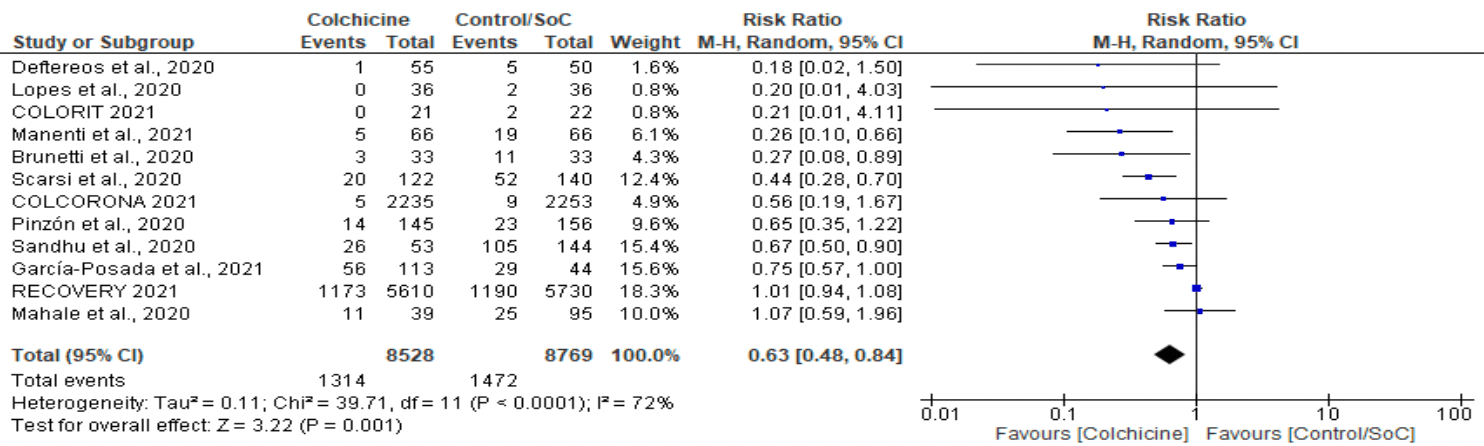
Meta analysis of 12 colchicine studies, showing significantly lower mortality with treatment.
10 meta-analyses show significant improvements with colchicine for mortality1-8,
oxygen therapy8,
hospitalization9, and
severity10.
Currently there are 54 colchicine for COVID-19 studies, showing 22% lower mortality [12‑31%], 29% lower ventilation [-15‑56%], 34% lower ICU admission [8‑52%], 17% lower hospitalization [9‑25%], and 9% more cases [-8‑29%].
|
risk of death, 37.0% lower, RR 0.63, p = 0.001.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zein et al., Effect of colchicine on mortality in patients with COVID-19 – A systematic review and meta-analysis, Diabetes & Metabolic Syndrome: Clinical Research & Reviews, doi:10.1016/j.dsx.2022.102395.
2.
Rai et al., The Potential Role of Colchicine in Reducing Mortality and Mechanical Ventilation Rates in COVID-19 Infection: A Meta-analysis, Journal of Advances in Medicine and Medical Research, doi:10.9734/jammr/2022/v34i2031503.
3.
Elshafei et al., Colchicine use might be associated with lower mortality in COVID‐19 patients: A meta‐analysis, European Journal of Clinical Investigation, doi:10.1111/eci.13645.
4.
Lien et al., Repurposing Colchicine in Treating Patients with COVID-19: A Systematic Review and Meta-Analysis, Life, doi:10.3390/life11080864.
5.
Danjuma et al., Does Colchicine Reduce Mortality in Patients with Covid-19 Clinical Syndrome? An Umbrella Review of Published Meta-Analyses, Elsevier BV, doi:10.2139/ssrn.4447127.
6.
Salah et al., Meta-analysis of the Effect of Colchicine on Mortality and Mechanical Ventilation in COVID-19, The American Journal of Cardiology, doi:10.1016/j.amjcard.2021.02.005.
7.
Golpour et al., The effectiveness of Colchicine as an anti-inflammatory drug in the treatment of coronavirus disease 2019: Meta-analysis, International Journal of Immunopathology and Pharmacology, doi:10.1177/20587384211031763.
8.
Elshiwy et al., The role of colchicine in the management of COVID-19: a meta-analysis, BMC Pulmonary Medicine, doi:10.1186/s12890-024-03001-0.
Rai et al., 27 Jul 2022, peer-reviewed, 15 authors.
Contact: iqra.mukhtar@live.com.
The Potential Role of Colchicine in Reducing Mortality and Mechanical Ventilation Rates in COVID-19 Infection: A Meta-analysis
Journal of Advances in Medicine and Medical Research, doi:10.9734/jammr/2022/v34i2031503
Background: Colchicine is one of many drugs being repurposed for COVID-19 due to its potential as an anti-inflammatory agent alongside its easy accessibility and oral administration. This study aims to identify the risk reduction in mortality and mechanical ventilation of colchicine-treated COVID-19 patients compared to the standard of care/placebo. Methods: A systematic search was conducted until December 31, 2021, with keywords including Colchicine, COVID-19, SARS-CoV-2, anti-inflammatory, trials, clinical, mechanical ventilation, death, and mortality. Databases including MEDLINE/PubMed, Scopus, Web of Science, CINAHL Plus, Cochrane, WHO Global Database, and Preprint servers were searched. Using dichotomous data for all values, the risk ratios (RR) were calculated by applying the random-effects model in Review Manager 5.4.
Results: The 12 studies pooled 17,297 participants, with 8,528 patients in the colchicine group and 8,769 in the standard care group. Colchicine treatment led to a statistically significant reduction in the risk of death (RR=0.63, 95% CI=0.48-0.84, P=0.001). Moderately high heterogeneity was present among the included studies (I 2 =72%). While insignificant, the risk of mechanical ventilation was decreased by 12% among the colchicine group (RR=0.88, 95% CI=0.64-1.22, P=0.44). Conclusions: While this meta-analysis finds overall reductions in mortality with colchicine treatment, these findings must be utilized with caution. Placebo-controlled randomized clinical trials are warranted at a large scale to validate the viability of colchicine as an adjuvant treatment for COVID-19. On obtaining more concrete findings, the potential role of colchicine may be better optimized in non-severe patients as well, across in-hospital and outpatient settings.
References
Brunetti, Diawara, Tsai, Colchicine to Weather the Cytokine Storm in Hospitalized Patients with COVID-19, J Clin Med, doi:10.3390/jcm9092961
García-Posada, Vesga, Mestra, Clinical outcomes of patients hospitalized for COVID-19 and evidence-based on the pharmacological management reduce mortality in a region of the Colombian Caribbean, J Infect Public Health, doi:10.1016/j.jiph.2021.02.013
Mahale, Rajhans, Godavarthy, A Retrospective Observational Study of Hypoxic COVID-19 Patients Treated with Immunomodulatory Drugs in a Tertiary Care Hospital, Indian J Crit Care Med, doi:10.5005/jp-journals-10071-23599
Manenti, Maggiore, Fiaccadori, Reduced mortality in COVID-19 patients treated with colchicine: Results from a retrospective, observational study, PLoS One, doi:10.1371/journal.pone.0248276
Mori, Taki, Wakabayashi, Colchicine treatment early after infarction attenuates myocardial inflammatory response demonstrated by 14 C-methionine imaging and subsequent ventricular remodeling by quantitative gated SPECT, Ann Nucl Med, doi:10.1007/s12149-020-01559-3
Pascart, Richette, Colchicine in Gout: An Update, Curr Pharm Des, doi:10.2174/13816128249991801151039
Reyes, Hu, Teperman, Antiinflammatory therapy for COVID-19 infection: the case for colchicine, Ann Rheum Dis, doi:10.1136/annrheumdis-2020-219174
Reyes, Hu, Teperman, Antiinflammatory therapy for COVID-19 infection: the case for colchicine, Ann Rheum Dis, doi:10.1136/annrheumdis-2020-219174
Romagnoli, Peris, Gaudio, Geppetti, SARS-CoV-2 and COVID-19: From the Bench to the Bedside, Physiol Rev, doi:10.1152/physrev.00020.2020
Sandhu, Tieng, Chilimuri, Franchin, A Case Control Study to Evaluate the Impact of Colchicine on Patients Admitted to the Hospital with Moderate to Severe COVID-19 Infection, Can J Infect Dis Med Microbiol, doi:10.1155/2020/8865954
Stewart, Yang, Atkins, Adverse events during oral colchicine
Van Echteld, Wechalekar, Schlesinger, Buchbinder, Aletaha, Colchicine for acute gout, Cochrane Database Syst Rev, doi:10.1002/14651858.CD006190.pub2
Vrachatis, Giannopoulos, Giotaki, Impact of colchicine on mortality in patients with COVID-19: A meta
Xia, Yang, Qian, Meta-analysis Evaluating the Utility of Colchicine in Secondary Prevention of Coronary Artery Disease, Am J Cardiol, doi:10.1016/j.amjcard.2020.10.043
DOI record:
{
"DOI": "10.9734/jammr/2022/v34i2031503",
"ISSN": [
"2456-8899"
],
"URL": "http://dx.doi.org/10.9734/jammr/2022/v34i2031503",
"abstract": "<jats:p>Background: Colchicine is one of many drugs being repurposed for COVID-19 due to its potential as an anti-inflammatory agent alongside its easy accessibility and oral administration. This study aims to identify the risk reduction in mortality and mechanical ventilation of colchicine-treated COVID-19 patients compared to the standard of care/placebo.
\nMethods: A systematic search was conducted until December 31, 2021, with keywords including Colchicine, COVID-19, SARS-CoV-2, anti-inflammatory, trials, clinical, mechanical ventilation, death, and mortality. Databases including MEDLINE/PubMed, Scopus, Web of Science, CINAHL Plus, Cochrane, WHO Global Database, and Preprint servers were searched. Using dichotomous data for all values, the risk ratios (RR) were calculated by applying the random-effects model in Review Manager 5.4.
\nResults: The 12 studies pooled 17,297 participants, with 8,528 patients in the colchicine group and 8,769 in the standard care group. Colchicine treatment led to a statistically significant reduction in the risk of death (RR=0.63, 95% CI=0.48-0.84, P=0.001). Moderately high heterogeneity was present among the included studies (I2=72%). While insignificant, the risk of mechanical ventilation was decreased by 12% among the colchicine group (RR=0.88, 95% CI=0.64-1.22, P=0.44).
\nConclusions: While this meta-analysis finds overall reductions in mortality with colchicine treatment, these findings must be utilized with caution. Placebo-controlled randomized clinical trials are warranted at a large scale to validate the viability of colchicine as an adjuvant treatment for COVID-19. On obtaining more concrete findings, the potential role of colchicine may be better optimized in non-severe patients as well, across in-hospital and outpatient settings.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Rai",
"given": "Shilpa",
"sequence": "first"
},
{
"affiliation": [],
"family": "Nijjar",
"given": "Shahbaz Singh",
"sequence": "first"
},
{
"affiliation": [],
"family": "T. OJinna",
"given": "Blessing",
"sequence": "first"
},
{
"affiliation": [],
"family": "Mukhtar",
"given": "Iqra",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ahmed",
"given": "Maryam",
"sequence": "first"
},
{
"affiliation": [],
"family": "Tobalesi",
"given": "Opeyemi",
"sequence": "first"
},
{
"affiliation": [],
"family": "Singh",
"given": "Prerna",
"sequence": "first"
},
{
"affiliation": [],
"family": "Adefashola",
"given": "Olamide",
"sequence": "first"
},
{
"affiliation": [],
"family": "O. Aboaba",
"given": "Abiodun",
"sequence": "first"
},
{
"affiliation": [],
"family": "Waqar",
"given": "Danish",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ndove",
"given": "Jeffrey",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ferguson",
"given": "Frederick",
"sequence": "first"
},
{
"affiliation": [],
"family": "Adedoyin",
"given": "Adewale Mark",
"sequence": "first"
},
{
"affiliation": [],
"family": "Batti",
"given": "Patrick",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zafar",
"given": "Hammad",
"sequence": "first"
}
],
"container-title": "Journal of Advances in Medicine and Medical Research",
"container-title-short": "JAMMR",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
7,
30
]
],
"date-time": "2022-07-30T04:40:59Z",
"timestamp": 1659156059000
},
"deposited": {
"date-parts": [
[
2022,
7,
30
]
],
"date-time": "2022-07-30T04:41:00Z",
"timestamp": 1659156060000
},
"indexed": {
"date-parts": [
[
2022,
7,
30
]
],
"date-time": "2022-07-30T05:13:00Z",
"timestamp": 1659157980483
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
7,
27
]
]
},
"link": [
{
"URL": "https://journaljammr.com/index.php/JAMMR/article/download/31503/59027",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journaljammr.com/index.php/JAMMR/article/download/31503/59028",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journaljammr.com/index.php/JAMMR/article/download/31503/59027",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "4694",
"original-title": [],
"page": "349-358",
"prefix": "10.9734",
"published": {
"date-parts": [
[
2022,
7,
27
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
27
]
]
},
"publisher": "Sciencedomain International",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://journaljammr.com/index.php/JAMMR/article/view/31503"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "The Potential Role of Colchicine in Reducing Mortality and Mechanical Ventilation Rates in COVID-19 Infection: A Meta-analysis",
"type": "journal-article"
}
