Colchicine to Weather the Cytokine Storm in Hospitalized Patients with COVID-19
et al., Journal of Clinical Medicine, doi:10.3390/jcm9092961, Sep 2020
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
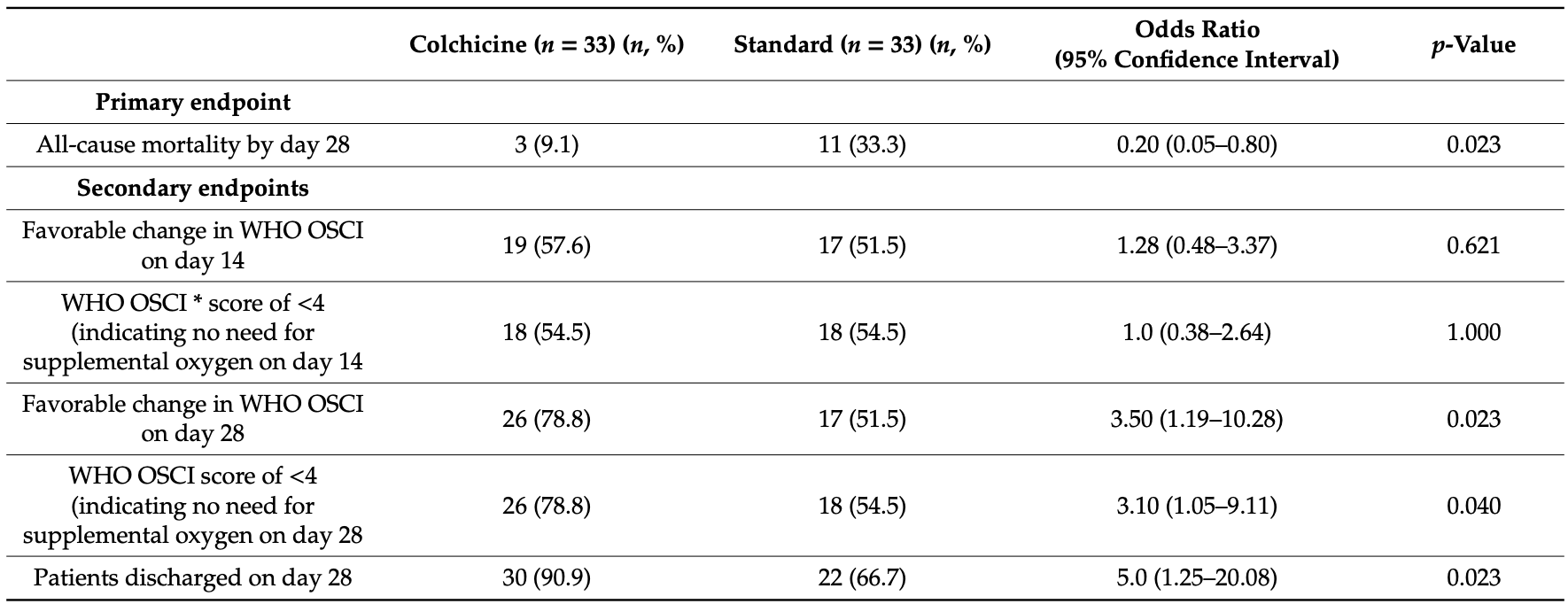
PSM matched analysis from consecutive hospitalized patients, with 33 colchicine and 33 control matched patients, showing lower mortality with treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
|
risk of death, 72.7% lower, RR 0.27, p = 0.03, treatment 3 of 33 (9.1%), control 11 of 33 (33.3%), NNT 4.1, PSM.
|
|
risk of no hospital discharge, 72.7% lower, RR 0.27, p = 0.03, treatment 3 of 33 (9.1%), control 11 of 33 (33.3%), NNT 4.1, PSM.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Brunetti et al., 14 Sep 2020, retrospective, propensity score matching, USA, peer-reviewed, baseline oxygen required 86.4%, 7 authors, dosage 1.2mg daily.
Colchicine to Weather the Cytokine Storm in Hospitalized Patients with COVID-19
Journal of Clinical Medicine, doi:10.3390/jcm9092961
The repurposing of colchicine for the treatment of COVID-19 was suggested based in its immunomodulatory, anti-inflammatory, and anti-viral properties. We performed a single-center propensity score matched cohort study, including all consecutive COVID-19 patients admitted to a community hospital between 1 March 2020 and 30 May 2020. Patients were stratified according to the receipt of colchicine. The primary endpoint was defined as in-hospital death within 28-days follow-up. Secondary endpoints included favorable change in the Ordinal Scale for Clinical Improvement on days 14 and 28 versus baseline, proportion of patients not requiring supplemental oxygen on days 14 and 28, and proportion of patients discharged by day 28. In total data for 303 PCR positive COVID-19 patients were extracted and 66 patients were included in the 1:1 matched cohort study. At the end of the 28 day follow-up, patients receiving colchicine were approximately five times more likely to be discharged (odds ratio, 5.0; 95% confidence interval, 1.25-20.1; p = 0.023) and when comparing mortality, there were 3 deaths (9.1%) in patients receiving colchicine versus 11 deaths (33.3%) in the groups receiving standard of care (odds ratio, 0.20; 95% confidence interval, 0.05-0.80; p = 0.023). These observations warrant further investigation in large controlled clinical trials.
Conflicts of Interest: The authors declare no conflict of interest.
References
Bhimraj, Morgan, Shumaker, Lavergne, Baden et al., Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19, Clin. Infect. Dis, doi:10.1093/cid/ciaa478
Cumhur Cure, Kucuk, Cure, Colchicine may not be effective in COVID-19 infection; it may even be harmful?, Clin. Rheumatol, doi:10.1007/s10067-020-05144-x
Deftereos, Giannopoulos, Vrachatis, Siasos, Giotaki et al., Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized With Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2020.13136
Della-Torre, Della-Torre, Kusanovic, Scotti, Ramirez et al., Treating COVID-19 with colchicine in community healthcare setting, Clin. Immunol, doi:10.1016/j.clim.2020.108490
Della-Torre, Ramirez, Dagna, Tresoldi, Colchicine treatment in community healthcare setting to prevent severe COVID-19, Ann. Rheum. Dis, doi:10.1136/annrheumdis-2020-218759
Deyo, Cherkin, Ciol, Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases, J. Clin. Epidemiol, doi:10.1016/0895-4356(92)90133-8
Fried, Ramasubbu, Bhatt, Topkara, Clerkin et al., The Variety of Cardiovascular Presentations of COVID-19, Circulation, doi:10.1161/CIRCULATIONAHA.120.047164
Gendelman, Amital, Bragazzi, Watad, Chodick, Continuous hydroxychloroquine or colchicine therapy does not prevent infection with SARS-CoV-2: Insights from a large healthcare database analysis, Autoimmun. Rev, doi:10.1016/j.autrev.2020.102566
Greber, Signalling in viral entry, Cell Mol. Life Sci, doi:10.1007/s00018-002-8453-3
Group, Horby, Lim, Emberson, Mafham et al., Dexamethasone in Hospitalized Patients with Covid-19-Preliminary Report, N. Engl. J. Med, doi:10.1056/NEJMoa2021436
Jackman, Rhoads, Cornwell, Kandarian, Microtubule-mediated NF-kappaB activation in the TNF-alpha signaling pathway, Exp. Cell Res, doi:10.1016/j.yexcr.2009.08.020
Parra-Medina, Sarmiento-Monroy, Rojas-Villarraga, Garavito, Montealegre-Gomez et al., Colchicine as a possible therapeutic option in COVID-19 infection, Clin. Rheumatol, doi:10.1007/s10067-020-05247-5
Piantoni, Patroni, Toniati, Furloni, Franceschini et al., Why not to use colchicine in COVID-19? An oldanti-inflammatory drug for a novel auto-inflammatory disease, Rheumatology, doi:10.1093/rheumatology/keaa217
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, JAMA, doi:10.1001/jama.2020.6775
Richter, Boldescu, Graf, Streicher, Dimoglo et al., Synthesis, Biological Evaluation, and Molecular Docking of Combretastatin and Colchicine Derivatives and their hCE1-Activated Prodrugs as Antiviral Agents, ChemMedChem, doi:10.1002/cmdc.201800641
Scarsi, Piantoni, Colombo, Airo, Richini et al., Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome, Ann. Rheum. Dis, doi:10.1136/annrheumdis-2020-217712
Schlesinger, Firestein, Brunetti, Colchicine in COVID-19: An Old Drug, New Use, Curr. Pharmacol. Rep, doi:10.1007/s40495-020-00225-6
Tardif, Kouz, Waters, Bertrand, Diaz et al., Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction, N. Engl. J. Med, doi:10.1056/NEJMoa1912388
Von Elm, Altman, Egger, Pocock, Gotzsche et al., Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies, BMJ, doi:10.1136/bmj.39335.541782.AD
DOI record:
{
"DOI": "10.3390/jcm9092961",
"ISSN": [
"2077-0383"
],
"URL": "http://dx.doi.org/10.3390/jcm9092961",
"abstract": "<jats:p>The repurposing of colchicine for the treatment of COVID-19 was suggested based in its immunomodulatory, anti-inflammatory, and anti-viral properties. We performed a single-center propensity score matched cohort study, including all consecutive COVID-19 patients admitted to a community hospital between 1 March 2020 and 30 May 2020. Patients were stratified according to the receipt of colchicine. The primary endpoint was defined as in-hospital death within 28-days follow-up. Secondary endpoints included favorable change in the Ordinal Scale for Clinical Improvement on days 14 and 28 versus baseline, proportion of patients not requiring supplemental oxygen on days 14 and 28, and proportion of patients discharged by day 28. In total data for 303 PCR positive COVID-19 patients were extracted and 66 patients were included in the 1:1 matched cohort study. At the end of the 28 day follow-up, patients receiving colchicine were approximately five times more likely to be discharged (odds ratio, 5.0; 95% confidence interval, 1.25–20.1; p = 0.023) and when comparing mortality, there were 3 deaths (9.1%) in patients receiving colchicine versus 11 deaths (33.3%) in the groups receiving standard of care (odds ratio, 0.20; 95% confidence interval, 0.05–0.80; p = 0.023). These observations warrant further investigation in large controlled clinical trials.</jats:p>",
"alternative-id": [
"jcm9092961"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-0565-6167",
"affiliation": [],
"authenticated-orcid": false,
"family": "Brunetti",
"given": "Luigi",
"sequence": "first"
},
{
"affiliation": [],
"family": "Diawara",
"given": "Oumou",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tsai",
"given": "Andrew",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1679-3565",
"affiliation": [],
"authenticated-orcid": false,
"family": "Firestein",
"given": "Bonnie L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nahass",
"given": "Ronald G.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9983-0386",
"affiliation": [],
"authenticated-orcid": false,
"family": "Poiani",
"given": "George",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1558-1202",
"affiliation": [],
"authenticated-orcid": false,
"family": "Schlesinger",
"given": "Naomi",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical Medicine",
"container-title-short": "JCM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
9,
14
]
],
"date-time": "2020-09-14T13:04:53Z",
"timestamp": 1600088693000
},
"deposited": {
"date-parts": [
[
2020,
9,
16
]
],
"date-time": "2020-09-16T15:12:31Z",
"timestamp": 1600269151000
},
"indexed": {
"date-parts": [
[
2024,
3,
23
]
],
"date-time": "2024-03-23T05:20:52Z",
"timestamp": 1711171252082
},
"is-referenced-by-count": 58,
"issue": "9",
"issued": {
"date-parts": [
[
2020,
9,
14
]
]
},
"journal-issue": {
"issue": "9",
"published-online": {
"date-parts": [
[
2020,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
9,
14
]
],
"date-time": "2020-09-14T00:00:00Z",
"timestamp": 1600041600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2077-0383/9/9/2961/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "2961",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2020,
9,
14
]
]
},
"published-online": {
"date-parts": [
[
2020,
9,
14
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"key": "ref2"
},
{
"DOI": "10.1093/cid/ciaa478",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"key": "ref4"
},
{
"DOI": "10.1161/CIRCULATIONAHA.120.047164",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1056/NEJMoa1912388",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1007/s40495-020-00225-6",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1007/s00018-002-8453-3",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1016/j.yexcr.2009.08.020",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1002/cmdc.201800641",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"key": "ref11"
},
{
"DOI": "10.1016/0895-4356(92)90133-8",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"key": "ref13",
"unstructured": "World Health Organization (WHO) R&D Blueprint Group\nhttps://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf"
},
{
"DOI": "10.1136/bmj.39335.541782.AD",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1007/s10067-020-05144-x",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1016/j.autrev.2020.102566",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1007/s10067-020-05247-5",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1093/rheumatology/keaa217",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1136/annrheumdis-2020-217712",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1136/annrheumdis-2020-218759",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1016/j.clim.2020.108490",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1001/jama.2020.6775",
"doi-asserted-by": "publisher",
"key": "ref23"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2077-0383/9/9/2961"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Colchicine to Weather the Cytokine Storm in Hospitalized Patients with COVID-19",
"type": "journal-article",
"volume": "9"
}
