Proactive anti-inflammatory therapy with colchicine in the treatment of advanced stages of new coronavirus infection. The first results of the COLORIT study
et al., Kardiologiia, doi:10.18087/cardio.2021.2.n1560, Feb 2021
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Small trial with 21 colchicine patients and 22 control patients in Russia, showing improved recovery with treatment. The trial was originally an RCT, however randomization to the control arm was stopped after 5 patients, and 17 retrospective patients were added for comparison.
|
risk of death, 79.6% lower, RR 0.20, p = 0.49, treatment 0 of 21 (0.0%), control 2 of 22 (9.1%), NNT 11, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
ΔSHOCS-COVID, 50.0% lower, RR 0.50, p = 0.06, treatment 21, control 22, ΔSHOCS-COVID score, primary outcome.
|
|
SHOCS-COVID, 71.4% lower, RR 0.29, p = 0.002, treatment 21, control 22, SHOCS-COVID score.
|
|
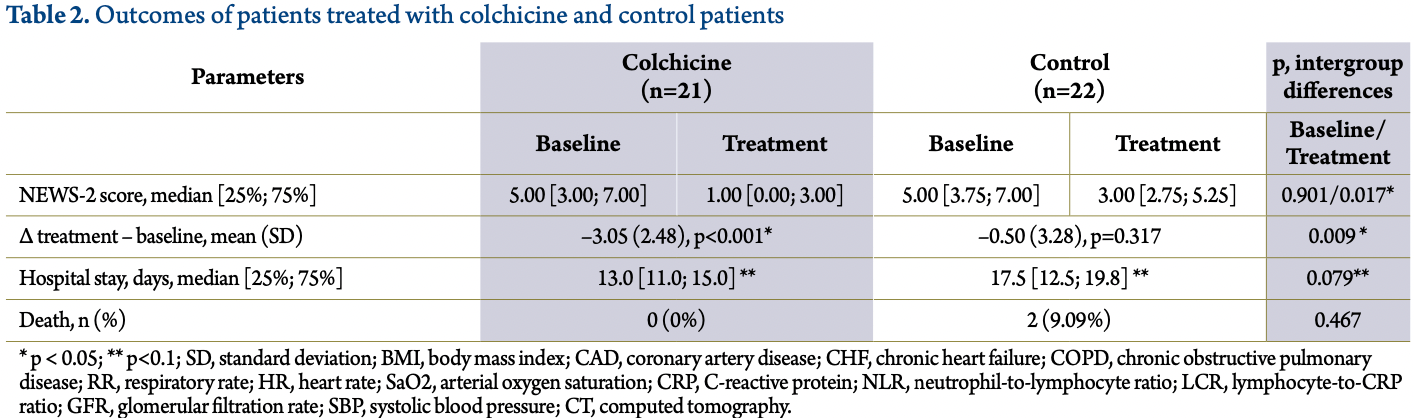
NEWS-2, 66.7% lower, RR 0.33, p = 0.06, treatment 21, control 22, inverted to make RR<1 favor treatment, NEWS-2 score.
|
|
hospitalization time, 25.7% lower, relative time 0.74, p = 0.08, treatment 21, control 22.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mareev et al., 28 Feb 2021, retrospective, Russia, peer-reviewed, 21 authors, dosage 1mg days 1-3.
Proactive anti-inflammatory therapy with colchicine in the treatment of advanced stages of new coronavirus infection. The first results of the COLORIT study
Kardiologiia, doi:10.18087/cardio.2021.2.n1560
Conclusions Colchicine 1 mg for 1-3 days followed by 0.5 mg / day for 14 days is effective as a proactive antiinflammatory therapy in hospitalized patients with COVID-19 and viral pneumonia. The management of such patients without proactive anti-inflammatory therapy is likely to be unreasonable and may worsen the course of COVID-19. However, the findings should be treated with caution, given the small size of the trial.
Additional materials Boxplot graphs with the dynamics of SHOCS-COVID, NEWS-2, SPO 2 and others are available in the section «Additional materials» to the article on the website of the journal.
Limitations of this study No appropriate randomization, few patients.
No conflict of interest is reported. The article was received on 08/02/2021 R EFER ENCES
References
Brunetti, Diawara, Tsai, Firestein, Nahass et al., Colchicine to Weather the Cytokine Storm in Hospitalized Patients with COVID-19, Journal of Clinical Medicine, doi:10.3390/jcm9092961
Deftereos, Giannopoulos, Vrachatis, Siasos, Giotaki et al., Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized with Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial, JAMA Network Open, doi:10.1001/jamanet-workopen.2020.13136
Demidowich, Davis, Dedhia, Yanovski, Colchicine to decrease NLRP3-activated inflammation and improve obesity-related metabolic dysregulation, Medical Hypotheses, doi:10.1016/j.mehy.2016.04.039
Fa, Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis, Journal of Medical Virology, doi:10.1002/jmv.25819
Feng, Yu, Yao, Luo, Zhou et al., Early prediction of disease progression in COVID-19 pneumonia patients EDITORIAL ARTICLES § with chest CT and clinical characteristics, Nature Communications, doi:10.1038/s41467-020-18786-x
Fitzgerald, Dalbeth, Mikuls, Brignardello-Petersen, Guyatt et al., American College of Rheumatology Guideline for the Management of Gout, Arthritis Care & Research, doi:10.1002/acr.24180
Kamalov, Mareev, Yu, Orlova Ya, Plisyk et al., Open comparative controlled trial on use of hydroxychloroquine for treatment of patients with novel coronavirus infection (COVID-19)
Karataş, İpek, Onuk, Güngör, Durmuş et al., Assessment of Prognostic Value of Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio in Patients with Pulmonary Embolism, Acta Cardiologica Sinica, doi:10.6515/acs20151013a
Kong, Zhang, Cao, Mao, Lu, Higher level of neutrophil-to-lymphocyte is associated with severe COVID-19. Epidemiology and, Infection, doi:10.1017/S0950268820001557
Landray, Colchicine for non-hospitalised pts with COV-ID-19
Li, Liu, Huang, Jia, Xia et al., Dynamic changes in clinical and CT characteristics of COVID-19 cases with different exposure histories: a retrospective study, BMC Infectious Diseases, doi:10.1186/s12879-020-05306-x
Liao, Wang, Kang, Novel coronavirus infection during the 2019-2020 epidemic: preparing intensive care units -the experience in Sichuan Province, China, Intensive Care Medicine, doi:10.1007/s00134-020-05954-2
Lu, Chen, Xiao, Li, Miller, An Overview of Tubulin Inhibitors That Interact with the Colchicine Binding Site, Pharmaceutical Research, doi:10.1007/s11095-012-0828-z
Mareev, Yu, Begrambekova, Mareev, Мареев, How evaluate results of treatment in patients with COVID-19? Symptomatic Hospital and Outpatient Clinical Scale for COV-ID-19 (SHOCS-COVID), Kardiologiia, doi:10.18087/cardio.2020.11.n1439
Mareev, Yu, Orlova Ya, Pavlikova, Akopyan Zh et al., Proactive anti-inflammatory and anticoagulant therapy in the treatment of advanced stages of novel coronavirus infection (COVID-19). Case Series and Study Design: COLchicine versus ruxolitinib and secukinumab in open prospective randomIzed trial (COLORIT), doi:10.18087/cardio.2020.9.n1338
Mareev, Yu, Orlova Ya, Pavlikova, Matskeplishvili et al., Combination therapy at an early stage of the novel coronavirus infection (C O VI D-19). Case series and design of the clinical trial "Brom-hexIne and Spironolactone for CoronаvirUs Infection requiring hospiTalization (BISCUIT), doi:10.18087/cardio.2020.8.n1307
Mareev, Yu, Orlova Ya, Pavlikova, Matskeplishvili et al., др. Пульс-терапия стероидными гормонами больных с коронавирусной пневмонией (COVID-19), системным воспалением и риском венозных тромбозов и тромбоэмболий (исследование ПУТНИК), Kardiologiia, doi:10.18087/cardio.2020.6.n1226
Mareev, Yu, Orlova Ya, Plisyk, Pavlikova et al., Результаты открытого проспективного контролируемого сравнительного исследования по лечению новой коронавирусной инфекции (COVID-19): Бромгексин EDITORIAL ARTICLES § И Спиронолактон для лечения КоронаВирусной Инфекции, Требующей госпитализации, Kardiologiia, doi:10.18087/cardio.2020.11.1440
Martínez, Celermajer, Patel, The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation, Atherosclerosis, doi:10.1016/j.atherosclerosis.2017.12.027
Montealegre-Gómez, Garavito, Gómez-López, Rojas-Villarraga, Parra-Medina, Colchicine: A potential therapeutic tool against COVID-19. Experience of 5 patients, Reumatología Clínica, doi:10.1016/j.reuma.2020.05.001
Nidorf, Fiolet, Mosterd, Eikelboom, Schut et al., Colchicine in Patients with Chronic Coronary Disease, New England Journal of Medicine, doi:10.1056/NEJMoa2021372
Tardif, Bouabdallaoui, Allier, Gaudet, Shah et al., Efficacy of Colchicine in Non-Hospitalized Patients with COVID-19, doi:10.1101/2021.01.26.21250494.2021
Tardif, Kouz, Waters, Bertrand, Diaz et al., Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction, New England Journal of Medicine, doi:10.1056/NEJ-Moa1912388
Ullah, Basyal, Tariq, Almas, Saeed et al., Lymphocyte-to-C-Reactive Protein Ratio: A Novel Predictor of Adverse Outcomes in COVID-19, Journal of Clinical Medicine Research, doi:10.14740/jocmr4227
Vernadsky, Yu, Medvedeva, Garbukov, Yu et al., Метаболическая визуализация рака молочной железы методом однофотонной эмиссионной компьютерной томографии с 99mTc-1-тио-d-глюкозой. Российский Электронный Журнал, Russian Electronic Journal of Radiology, doi:10.21569/2222-7415-2019-9-4-82-96
Yang, Lv, Liu, Zhang, Zhang et al., Colchicine Alleviates Cholesterol Crystal-Induced Endothelial Cell Pyroptosis through Activating AMPK/SIRT1 Pathway. Oxidative Medicine and Cellular Longevity, doi:10.1155/2020/9173530
Yu, Yu, Matveev, Sychev, Current and future use of colchicine in patients with COVID-19
Zhang, Wu, Du, Luo, Hou et al., Neutrophilto-Lymphocyte Ratio may Replace Chest Computed Tomography to Reflect the Degree of Lung Injury in Patients with Corona Virus Disease, doi:10.21203/rs.3.rs-23201/v1.2020
Zhao, Zhong, Xie, Yu, Liu, CT Scans of Patients with 2019 Novel Coronavirus (COVID-19) Pneumonia. Theranostics, doi:10.7150/thno.45016
DOI record:
{
"DOI": "10.18087/cardio.2021.2.n1560",
"ISSN": [
"2412-5660",
"0022-9040"
],
"URL": "http://dx.doi.org/10.18087/cardio.2021.2.n1560",
"abstract": "<jats:p><jats:italic>Actuality</jats:italic> The course of the novel coronavirus disease (COVID-19) is unpredictable. It manifests in some cases as increasing inflammation to even the onset of a cytokine storm and irreversible progression of acute respiratory syndrome, which is associated with the risk of death in patients. Thus, proactive anti-inflammatory therapy remains an open serious question in patients with COVID-19 and pneumonia, who still have signs of inflammation on days 7–9 of the disease: elevated C-reactive protein (CRP)>60 mg/dL and at least two of the four clinical signs: fever >37.5°C; persistent cough; dyspnea (RR >20 brpm) and/or reduced oxygen blood saturation <94% when breathing atmospheric air. We designed the randomized trial: COLchicine versus Ruxolitinib and Secukinumab in Open-label Prospective Randomized Trial in Patients with COVID-19 (COLORIT). We present here data comparing patients who received colchicine with those who did not receive specific anti-inflammatory therapy. Results of the comparison of colchicine, ruxolitinib, and secukinumab will be presented later.<jats:italic>Objective</jats:italic> Compare efficacy and safety of colchicine compared to the management of patients with COVID-19 without specific anti-inflammatory therapy.<jats:italic>Material and Methods</jats:italic> Initially, 20 people were expected to be randomized in the control group. However, enrollment to the control group was discontinued subsequently after the inclusion of 5 patients due to the risk of severe deterioration in the absence of anti-inflammatory treatment. Therefore, 17 patients, who had not received anti-inflammatory therapy when treated in the MSU Medical Research and Educational Center before the study, were also included in the control group. The effects were assessed on day 12 after the inclusion or at discharge if it occurred earlier than on day 12. The primary endpoint was the changes in the SHOCS-COVID score, which includes the assessment of the patient’s clinical condition, CT score of the lung tissue damage, the severity of systemic inflammation (CRP changes), and the risk of thrombotic complications (D-dimer) [1].<jats:italic>Results</jats:italic> The median SHOCS score decreased from 8 to 2 (p = 0.017), i.e., from moderate to mild degree, in the colchicine group. The change in the SHOCS-COVID score was minimal and statistically insignificant in the control group. In patients with COVID-19 treated with colchicine, the CRP levels decreased rapidly and normalized (from 99.4 to 4.2 mg/dL, p<0.001). In the control group, the CRP levels decreased moderately and statistically insignificantly and achieved 22.8 mg/dL by the end of the follow-up period, which was still more than four times higher than normal. The most informative criterion for inflammation lymphocyte-to-C-reactive protein ratio (LCR) increased in the colchicine group by 393 versus 54 in the control group (p = 0.003). After treatment, it was 60.8 in the control group, which was less than 100 considered safe in terms of systemic inflammation progression. The difference from 427 in the colchicine group was highly significant (p = 0.003).The marked and rapid decrease in the inflammation factors was accompanied in the colchicine group by the reduced need for oxygen support from 14 (66.7%) to 2 (9.5%). In the control group, the number of patients without anti-inflammatory therapy requiring oxygen support remained unchanged at 50%. There was a trend to shorter hospital stays in the group of specific anti-inflammatory therapy up to 13 days compared to 17.5 days in the control group (p = 0.079). Moreover, two patients died in the control group, and there were no fatal cases in the colchicine group. In the colchicine group, one patient had deep vein thrombosis with D-dimer elevated to 5.99 µg/mL, which resolved before discharge.<jats:italic>Conclusions</jats:italic> Colchicine 1 mg for 1-3 days followed by 0.5 mg/day for 14 days is effective as a proactive anti-inflammatory therapy in hospitalized patients with COVID-19 and viral pneumonia. The management of such patients without proactive anti-inflammatory therapy is likely to be unreasonable and may worsen the course of COVID-19. However, the findings should be treated with caution, given the small size of the trial.</jats:p>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7285-2048",
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia\r\nFaculty of Fundamental Medicine, Lomonosov Moscow State University, Russia"
}
],
"authenticated-orcid": true,
"family": "Mareev",
"given": "V. Yu.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-8160-5612",
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia\r\nFaculty of Fundamental Medicine, Lomonosov Moscow State University, Russia"
}
],
"authenticated-orcid": true,
"family": "Orlova",
"given": "Ya. A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia\r\nFaculty of Fundamental Medicine, Lomonosov Moscow State University, Russia"
}
],
"family": "Plisyk",
"given": "A. G.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7693-5281",
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia\r\nFaculty of Fundamental Medicine, Lomonosov Moscow State University, Russia"
}
],
"authenticated-orcid": true,
"family": "Pavlikova",
"given": "E. P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia\r\nFaculty of Fundamental Medicine, Lomonosov Moscow State University, Russia"
}
],
"family": "Akopyan",
"given": "Z. A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia"
}
],
"family": "Matskeplishvili",
"given": "S. T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia"
}
],
"family": "Malakhov",
"given": "P. S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia\r\nFaculty of Fundamental Medicine, Lomonosov Moscow State University, Russia"
}
],
"family": "Krasnova",
"given": "T. N.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1490-2078",
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia\r\nFaculty of Fundamental Medicine, Lomonosov Moscow State University, Russia"
}
],
"authenticated-orcid": true,
"family": "Seredenina",
"given": "E. M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9820-4276",
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia\r\nFaculty of Fundamental Medicine, Lomonosov Moscow State University, Russia"
}
],
"authenticated-orcid": true,
"family": "Potapenko",
"given": "A. V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia\r\nFaculty of Fundamental Medicine, Lomonosov Moscow State University, Russia"
}
],
"family": "Agapov",
"given": "M. A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1939-7189",
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia"
}
],
"authenticated-orcid": true,
"family": "Asratyan",
"given": "D. A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia\r\nFaculty of Fundamental Medicine, Lomonosov Moscow State University, Russia"
}
],
"family": "Dyachuk",
"given": "L. I.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6734-3989",
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia\r\nFaculty of Fundamental Medicine, Lomonosov Moscow State University, Russia"
}
],
"authenticated-orcid": true,
"family": "Samokhodskaya",
"given": "L. M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1266-4926",
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia\r\nFaculty of Fundamental Medicine, Lomonosov Moscow State University, Russia"
}
],
"authenticated-orcid": true,
"family": "Mershina",
"given": "Е. А.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5649-2193",
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia\r\nFaculty of Fundamental Medicine, Lomonosov Moscow State University, Russia"
}
],
"authenticated-orcid": true,
"family": "Sinitsyn",
"given": "V. E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Fundamental Medicine, Lomonosov Moscow State University, Russia"
}
],
"family": "Pakhomov",
"given": "P. V.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7992-6081",
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia\r\nFaculty of Fundamental Medicine, Lomonosov Moscow State University, Russia"
}
],
"authenticated-orcid": true,
"family": "Zhdanova",
"given": "E. A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1939-7189",
"affiliation": [
{
"name": "National Medical Research Centre for Therapy and Preventive Medicine, Moscow, Russia\r\nRobertson Centre for Biostatistics, Glasgow, Great Britain"
}
],
"authenticated-orcid": true,
"family": "Mareev",
"given": "Yu. V.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7992-6081",
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia\r\nFaculty of Fundamental Medicine, Lomonosov Moscow State University, Russia"
}
],
"authenticated-orcid": true,
"family": "Begrambekova",
"given": "Yu. L.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4251-7545",
"affiliation": [
{
"name": "Medical Research and Educational Center of the M. V. Lomonosov Moscow State University, Moscow, Russia\r\nFaculty of Fundamental Medicine, Lomonosov Moscow State University, Russia"
}
],
"authenticated-orcid": true,
"family": "Kamalov",
"given": "А. А.",
"sequence": "additional"
}
],
"container-title": [
"Kardiologiia"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
3,
15
]
],
"date-time": "2021-03-15T06:53:07Z",
"timestamp": 1615791187000
},
"deposited": {
"date-parts": [
[
2021,
3,
15
]
],
"date-time": "2021-03-15T06:56:02Z",
"timestamp": 1615791362000
},
"indexed": {
"date-parts": [
[
2022,
2,
3
]
],
"date-time": "2022-02-03T03:26:04Z",
"timestamp": 1643858764463
},
"is-referenced-by-count": 15,
"issn-type": [
{
"type": "electronic",
"value": "2412-5660"
},
{
"type": "print",
"value": "0022-9040"
}
],
"issue": "2",
"issued": {
"date-parts": [
[
2021,
2,
28
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2021,
2,
28
]
]
}
},
"license": [
{
"URL": "https://lib.ossn.ru/jour/about/editorialPolicies#openAccessPolicy",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
2,
28
]
],
"date-time": "2021-02-28T00:00:00Z",
"timestamp": 1614470400000
}
}
],
"link": [
{
"URL": "https://lib.ossn.ru/jour/article/viewFile/1560/911",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://lib.ossn.ru/jour/article/viewFile/1560/932",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "7442",
"original-title": [],
"page": "15-27",
"prefix": "10.18087",
"published": {
"date-parts": [
[
2021,
2,
28
]
]
},
"published-online": {
"date-parts": [
[
2021,
2,
28
]
]
},
"publisher": "APO Society of Specialists in Heart Failure",
"reference-count": 34,
"references-count": 34,
"relation": {},
"score": 1,
"short-container-title": [
"Kardiologiia"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Cardiology and Cardiovascular Medicine"
],
"subtitle": [],
"title": [
"Proactive anti-inflammatory therapy with colchicine in the treatment of advanced stages of new coronavirus infection. The first results of the COLORIT study"
],
"type": "journal-article",
"volume": "61"
}
