Real-world effectiveness of molnupiravir, nirmatrelvir-ritonavir, and sotrovimab on preventing hospital admission among higher-risk patients with COVID-19 in Wales: A retrospective cohort study
et al., Journal of Infection, doi:10.1016/j.jinf.2023.02.012, Jan 2023
Retrospective high risk outpatients in the UK, showing lower hospitalization/death with molnupiravir treatment. Residual confounding is likely with adjustments having no detail on specific comorbidities.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments25.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
Study covers molnupiravir, sotrovimab, and paxlovid.
|
risk of death/hospitalization, 51.0% lower, HR 0.49, p = 0.008, treatment 359, control 4,973, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
23.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Evans et al., 25 Jan 2023, retrospective, United Kingdom, peer-reviewed, 11 authors, study period 16 December, 2021 - 22 April, 2022.
Contact: andrew.evans@gov.wales, cathy.qi@swansea.ac.uk, j.o.adebayo@swansea.ac.uk, underwoodj7@cardiff.ac.uk, coulsonjm@cardiff.ac.uk, r.bailey@swansea.ac.uk, gareth.john@wales.nhs.uk, edwardsag@cardiff.ac.uk, coopera8@cardiff.ac.uk, r.a.lyons@swansea.ac.uk, a.akbari@swansea.ac.uk.
Real-world effectiveness of molnupiravir, nirmatrelvir-ritonavir, and sotrovimab on preventing hospital admission among higher-risk patients with COVID-19 in Wales: A retrospective cohort study
Journal of Infection, doi:10.1016/j.jinf.2023.02.012
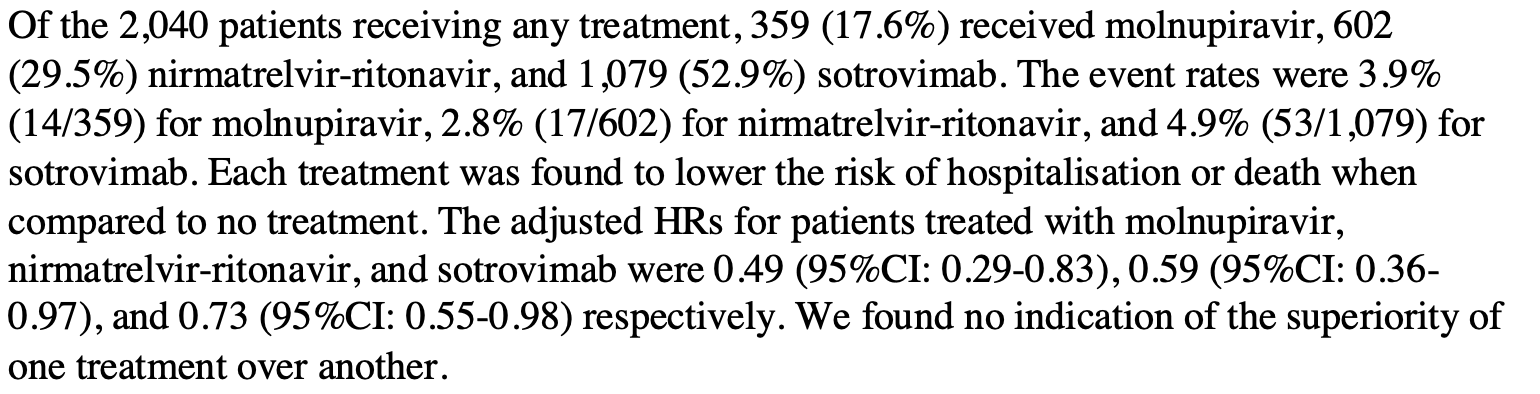
Objective: To compare the effectiveness of molnupiravir, nirmatrelvir-ritonavir, and sotrovimab with no treatment in preventing hospital admission or death in higher-risk patients infected with SARS-CoV-2 in the community. Design: Retrospective cohort study of non-hospitalized adult patients with COVID-19 using the Secure Anonymised Information Linkage (SAIL) Databank. Setting: A real-world cohort study was conducted within the SAIL Databank (a secure trusted research environment containing anonymised, individual, population-scale electronic health record (EHR) data) for the population of Wales, UK. Participants: Adult patients with COVID-19 in the community, at higher risk of hospitalization and death, testing positive for SARS-CoV-2 between 16th December 2021 and 22nd April 2022. Interventions: Molnupiravir, nirmatrelvir-ritonavir, and sotrovimab given in the community by local health boards and the National Antiviral Service in Wales. Main outcome measures: All-cause admission to hospital or death within 28 days of a positive test for SARS-CoV-2. Statistical analysis: Cox proportional hazard model with treatment status (treated/untreated) as a timedependent covariate and adjusted for age, sex, number of comorbidities, Welsh Index of Multiple Deprivation, and vaccination status. Secondary subgroup analyses were by treatment type, number of comorbidities, and before and on or after 20th February 2022, when omicron BA.1 and omicron BA.2 were the dominant subvariants in Wales. Results: Between 16th December 2021 and 22nd April 2022, 7013 higher-risk patients were eligible for inclusion in the study. Of these, 2040 received treatment with molnupiravir (359, 17.6%), nirmatrelvir-ritonavir (602, 29.5%), or sotrovimab (1079, 52.9%). Patients in the treatment group were younger (mean age 53 vs 57 years), had fewer comorbidities, and a higher proportion had received four or more doses of the COVID-19 vaccine (36.3% vs 17.6%). Within 28 days of a positive test, 628 (9.0%) patients were admitted to hospital or died (84 treated and 544 untreated). The primary analysis indicated a lower risk of hospitalization or death at any point within 28 days in treated participants compared to those not receiving treatment. The adjusted hazard rate was 35% (95% CI: 18-49%) lower in treated than untreated participants. There was no indication of the superiority of one treatment over another and no evidence of a reduction in Journal of Infection xxx (xxxx) xxx-xxx
Ethics approval This project uses anonymised individual-level data sources held within the Trusted Research Environment provided by the SAIL Databank at Swansea University, Swansea, UK. All proposals to use SAIL data are subject to review by the independent Information Governance Review Panel (IGRP). This work was approved under proposal number 0911 after careful considerations by IGRP Panel. Access to data is gained through a privacy-protecting safe haven and remote access system referred to as the SAIL Gateway.
CRediT authorship contribution statement All authors were involved in the Conceptualization and design of the study. AE, GJ, AA, CQ, LA and RB contributed to the acquisition of data. CQ, LA and RB carried out the statistical analysis. AE, CQ and LA drafted the original version of the manuscript. All authors contributed to the interpretation of the results and critical revision of the manuscript. All authors approved the final manuscript. AE is the guarantor for this study. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Reporting We used the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) checklist to guide our transparent reporting of our work. A completed STROBE Statement -Checklist of items that should be included in reports of cohort studies is provided.
Appendix A. Supplementary material Supplementary data associated with this article..
References
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med
Butler, Hobbs, Gbinigie, Rahman, Hayward et al., Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet, doi:10.1016/S0140-6736(22)02597-1
Cheng, Reyes, Satram, Birch, Gibbons et al., Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-CoV-2 Delta and Omicron waves in the United States
Cox, Peacock, Harvey, Hughes, Wright, SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies, Nat Rev Microbiol
Ford, Jones, Verplanke, Lyons, Brown, The SAIL databank: building a national architecture for e-health research and evaluation, BMC Health Serv Res
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early remdesivir to prevent progression to severe Covid-19 in outpatients, N Engl J Med
Government, Coronavirus (COVID-19) infection survey (positivity estimates
Government, Weekly COVID-19 treatment counts by therapeutic agent in Wales
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med
Jones, Ford, Jones, Dsilva, Thompson et al., A case study of the secure anonymous information linkage (SAIL) gateway: a privacy protecting remote access system for health related research and evaluation, J Biomed Inform
Koslov, Merck's COVID pill loses its lustre: what that means for the pandemic, Nature, doi:10.1038/d41586-021-03667-0
Lyons, Jones, John, Brooks, Verplanke et al., The SAIL databank: linking multiple health and social care datasets, BMC Med Inform Decis Mak
Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients, Clin Infect Dis
Nhs England, COVID-19 therapeutics (antivirals, neutralising monoclonal antibodies and interleukin 6 inhibitors)
Nhs England, Interim clinical commissioning policy: treatments for non-hospitalised patients with COVID-19
Patel, Yarwood, Levick, Gibbons, Drysdale et al., Characteristics and outcomes of patients with COVID-19 at high-risk of disease progression receiving sotrovimab, oral antivirals or no treatment in England, doi:10.1101/2022.11.28.22282808
Piccicacco, Zeitler, Ing, Montero, Faughn et al., Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the Omicron surge, J Antimicrob Chemother
Radcliffe, Palacios, Azar, Cohen, Malinis, Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge, Am J Transplant
Roche, Ronapreve does not retain neutralising activity against the Omicron variant
Rodgers, Demmler, Dsilva, Lyons, Protecting health data privacy while using residence-based environment and demographic data, Health Place
Rodgers, Lyons, Dsilva, Jones, Brooks et al., Residential anonymous linking fields (RALFs): a novel information infrastructure to study the interaction between the environment and individuals' health, J Public Health
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med
Wu, Carr, Harvey, Mears, Kjaer et al., WHO's therapeutics and COVID-19 living guideline on mAbs needs to be reassessed, Lancet, doi:10.1016/S0140-6736(22)01938-9
Zheng, Green, Tazare, Curtis, Fisher et al., Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform, BMJ
DOI record:
{
"DOI": "10.1016/j.jinf.2023.02.012",
"ISSN": [
"0163-4453"
],
"URL": "http://dx.doi.org/10.1016/j.jinf.2023.02.012",
"alternative-id": [
"S0163445323000828"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Real-world effectiveness of molnupiravir, nirmatrelvir-ritonavir, and sotrovimab on preventing hospital admission among higher-risk patients with COVID-19 in Wales: A retrospective cohort study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jinf.2023.02.012"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "Crown Copyright © 2023 Published by Elsevier Ltd on behalf of The British Infection Association. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Evans",
"given": "Andrew",
"sequence": "first"
},
{
"affiliation": [],
"family": "Qi",
"given": "Cathy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adebayo",
"given": "Jubril Omololu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Underwood",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Coulson",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bailey",
"given": "Rowena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lyons",
"given": "Ronan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Edwards",
"given": "Adrian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cooper",
"given": "Alison",
"sequence": "additional"
},
{
"affiliation": [],
"family": "John",
"given": "Gareth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Akbari",
"given": "Ashley",
"sequence": "additional"
}
],
"container-title": "Journal of Infection",
"container-title-short": "Journal of Infection",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"journalofinfection.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
2,
10
]
],
"date-time": "2023-02-10T10:47:06Z",
"timestamp": 1676026026000
},
"deposited": {
"date-parts": [
[
2023,
2,
24
]
],
"date-time": "2023-02-24T15:41:42Z",
"timestamp": 1677253302000
},
"indexed": {
"date-parts": [
[
2023,
2,
25
]
],
"date-time": "2023-02-25T05:30:36Z",
"timestamp": 1677303036165
},
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2023,
2
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
2,
1
]
],
"date-time": "2023-02-01T00:00:00Z",
"timestamp": 1675209600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0163445323000828?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0163445323000828?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
2
]
]
},
"published-print": {
"date-parts": [
[
2023,
2
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.jinf.2023.02.012_bib1",
"unstructured": "Department of Health and Social Care. Government launches COVID-19 antivirals taskforce to roll out innovative home treatments this autumn; 2021 [cited 2023 Jan 20]. Available from: 〈https://www.gov.uk/government/news/government-launches-covid-19-antivirals-taskforce-to-roll-out-innovative-home-treatments-this-autumn〉."
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2023.02.012_bib2",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2023.02.012_bib3",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2023.02.012_bib4",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early remdesivir to prevent progression to severe Covid-19 in outpatients",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2023.02.012_bib5",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2108163",
"article-title": "REGEN-COV antibody combination and outcomes in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"issue": "23",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2023.02.012_bib6",
"volume": "385",
"year": "2021"
},
{
"article-title": "COVID-19 Genomics UK Consortium,et al., SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies",
"author": "Cox",
"first-page": "1",
"journal-title": "Nat Rev Microbiol",
"key": "10.1016/j.jinf.2023.02.012_bib7",
"year": "2022"
},
{
"key": "10.1016/j.jinf.2023.02.012_bib8",
"unstructured": "Roche. Ronapreve does not retain neutralising activity against the Omicron variant; 2021 [cited 2023 Jan 20] [internet]. Available from: 〈2021216_roche-statement-on-ronapreve-omicron.pdf〉."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib9",
"unstructured": "US Federal Drugs Administration. Coronavirus (COVID-19) update: FDA limits use of certain monoclonal antibodies to treat COVID-19 due to the Omicron variant; 2022 [cited 2023 Jan 20] [internet]. Available from: 〈https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-certain-monoclonal-antibodies-treat-covid-19-due-omicron〉."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib10",
"unstructured": "World Health Organisation. Therapeutics and COVID-19: living guideline; 2022 [cited 2023 Jan 20] [internet]. Available from: 〈https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.5〉."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib11",
"unstructured": "Department of Health and Social Care. Highest-risk patients eligible for new COVID-19 treatments: a guide for patients; 2022 [cited 2023 Jan 20]. Available from: 〈https://www.gov.uk/government/publications/highest-risk-patients-eligible-for-covid-19-treatments-guide-for-patients/highest-risk-patients-eligible-for-new-covid-19-treatments-a-guide-for-patients〉."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib12",
"unstructured": "NHS England. Interim clinical commissioning policy: treatments for non-hospitalised patients with COVID-19; 2022 [cited 2023 Jan 20] [internet]. Available from: 〈C1710-interim-clinical-commissioning-policy-treatments-for-non-hospitalised-patients-with-covid-19-nov-22.pdf〉 (england.nhs.uk)."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib13",
"unstructured": "NHS England. COVID-19 therapeutics (antivirals, neutralising monoclonal antibodies and interleukin 6 inhibitors); 2022 [cited 2023 Jan 20] [internet]. Available from: 〈https://www.england.nhs.uk/statistics/statistical-work-areas/covid-therapeutics-antivirals-and-neutralising-monoclonal-antibodies/〉."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib14",
"unstructured": "Welsh Government. Weekly COVID-19 treatment counts by therapeutic agent in Wales; 2022 [cited 2023 Jan 20] [internet]. Available from: 〈https://statswales.gov.wales/Catalogue/Health-and-Social-Care/coronavirus-covid-19/covid-19-treatments-by-therapeutic-agent/weeklycovidtreatmentcounts-by-therapeuticagent〉."
},
{
"DOI": "10.1186/1472-6963-9-157",
"article-title": "The SAIL databank: building a national architecture for e-health research and evaluation",
"author": "Ford",
"doi-asserted-by": "crossref",
"first-page": "157",
"journal-title": "BMC Health Serv Res",
"key": "10.1016/j.jinf.2023.02.012_bib15",
"volume": "9",
"year": "2009"
},
{
"DOI": "10.1016/j.jbi.2014.01.003",
"article-title": "A case study of the secure anonymous information linkage (SAIL) gateway: a privacy protecting remote access system for health related research and evaluation",
"author": "Jones",
"doi-asserted-by": "crossref",
"first-page": "196",
"issue": "100",
"journal-title": "J Biomed Inform",
"key": "10.1016/j.jinf.2023.02.012_bib16",
"volume": "50",
"year": "2014"
},
{
"DOI": "10.1186/1472-6947-9-3",
"article-title": "The SAIL databank: linking multiple health and social care datasets",
"author": "Lyons",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "BMC Med Inform Decis Mak",
"key": "10.1016/j.jinf.2023.02.012_bib17",
"volume": "9",
"year": "2009"
},
{
"DOI": "10.1016/j.healthplace.2011.09.006",
"article-title": "Protecting health data privacy while using residence-based environment and demographic data",
"author": "Rodgers",
"doi-asserted-by": "crossref",
"first-page": "209",
"issue": "2",
"journal-title": "Health Place",
"key": "10.1016/j.jinf.2023.02.012_bib18",
"volume": "18",
"year": "2012"
},
{
"DOI": "10.1093/pubmed/fdp041",
"article-title": "Residential anonymous linking fields (RALFs): a novel information infrastructure to study the interaction between the environment and individuals’ health",
"author": "Rodgers",
"doi-asserted-by": "crossref",
"first-page": "582",
"issue": "4",
"journal-title": "J Public Health",
"key": "10.1016/j.jinf.2023.02.012_bib19",
"volume": "31",
"year": "2009"
},
{
"key": "10.1016/j.jinf.2023.02.012_bib20",
"unstructured": "Welsh Government. Coronavirus (COVID-19) infection survey (positivity estimates): 27 March to 2 April 2022; 2022 [cited 2023 Jan 20] [internet]. Available from: 〈https://www.gov.wales/coronavirus-covid-19-infection-survey-positivity-estimates-27-march-2-april-2022-html〉."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib21",
"unstructured": "Welsh Medicines Information Centre. National Antiviral Service Cymru (NAVS) – information for members of the public; 2022 [cited 2023 Jan 20]. Available from: 〈https://www.wmic.wales.nhs.uk/navs-cymru/#:~:text=%E2%80%93%20Information%20for%20Members%20of%20the,not%20been%20admitted%20to%20hospital〉."
},
{
"article-title": "Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients",
"author": "Najjar-Debbiny",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jinf.2023.02.012_bib22",
"year": "2022"
},
{
"DOI": "10.1101/2022.09.07.22279497",
"doi-asserted-by": "crossref",
"key": "10.1016/j.jinf.2023.02.012_bib23",
"unstructured": "Cheng M, Reyes C, Satram S, H. Birch, D.C. Gibbons, M. Drysdale, et al. Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-CoV-2 Delta and Omicron waves in the United States. [Preprint]. medRxiv 2022.09.07.22279497."
},
{
"DOI": "10.1093/jac/dkac256",
"article-title": "Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the Omicron surge",
"author": "Piccicacco",
"doi-asserted-by": "crossref",
"first-page": "2693",
"issue": "10",
"journal-title": "J Antimicrob Chemother",
"key": "10.1016/j.jinf.2023.02.012_bib24",
"volume": "77",
"year": "2022"
},
{
"article-title": "Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial",
"author": "Butler",
"journal-title": "Lancet",
"key": "10.1016/j.jinf.2023.02.012_bib25",
"year": "2022"
},
{
"article-title": "Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform",
"author": "Zheng",
"journal-title": "BMJ",
"key": "10.1016/j.jinf.2023.02.012_bib26",
"volume": "379",
"year": "2022"
},
{
"article-title": "Characteristics and outcomes of patients with COVID-19 at high-risk of disease progression receiving sotrovimab, oral antivirals or no treatment in England",
"author": "Patel",
"journal-title": "medRxiv",
"key": "10.1016/j.jinf.2023.02.012_bib27",
"year": "2022"
},
{
"DOI": "10.1111/ajt.17098",
"article-title": "Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge",
"author": "Radcliffe",
"doi-asserted-by": "crossref",
"first-page": "2458",
"journal-title": "Am J Transplant",
"key": "10.1016/j.jinf.2023.02.012_bib28",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)01938-9",
"article-title": "WHO’s therapeutics and COVID-19 living guideline on mAbs needs to be reassessed",
"author": "Wu",
"doi-asserted-by": "crossref",
"journal-title": "Lancet",
"key": "10.1016/j.jinf.2023.02.012_bib29",
"year": "2022"
},
{
"DOI": "10.1038/d41586-021-03667-0",
"article-title": "Merck’s COVID pill loses its lustre: what that means for the pandemic",
"author": "Koslov",
"doi-asserted-by": "crossref",
"journal-title": "Nature",
"key": "10.1016/j.jinf.2023.02.012_bib30",
"year": "2021"
},
{
"key": "10.1016/j.jinf.2023.02.012_bib31",
"unstructured": "Office for National Statistics. Coronavirus (COVID-19) latest insights: hospitals; 2023 [cited 2023 Jan 20] [internet]. Available from: 〈https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/hospitals〉."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib32",
"unstructured": "The National Institute for Health and Care Excellence. Draft guidance consultation: therapeutics for people with COVID-19; 2022 [cited 2023 Jan 20] [internet]. Available from: 〈https://www.nice.org.uk/guidance/gid-ta10936/documents/129〉."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib33",
"unstructured": "The Institute for Clinical and Economic Review. Report at a glance: COVID-19; 2022 [cited 2023 Jan 20]. Available from: 〈https://icer.org/wp-content/uploads/2022/05/COVID-19-RAAG_10May2022.pdf〉."
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0163445323000828"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Real-world effectiveness of molnupiravir, nirmatrelvir-ritonavir, and sotrovimab on preventing hospital admission among higher-risk patients with COVID-19 in Wales: A retrospective cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}
evans2