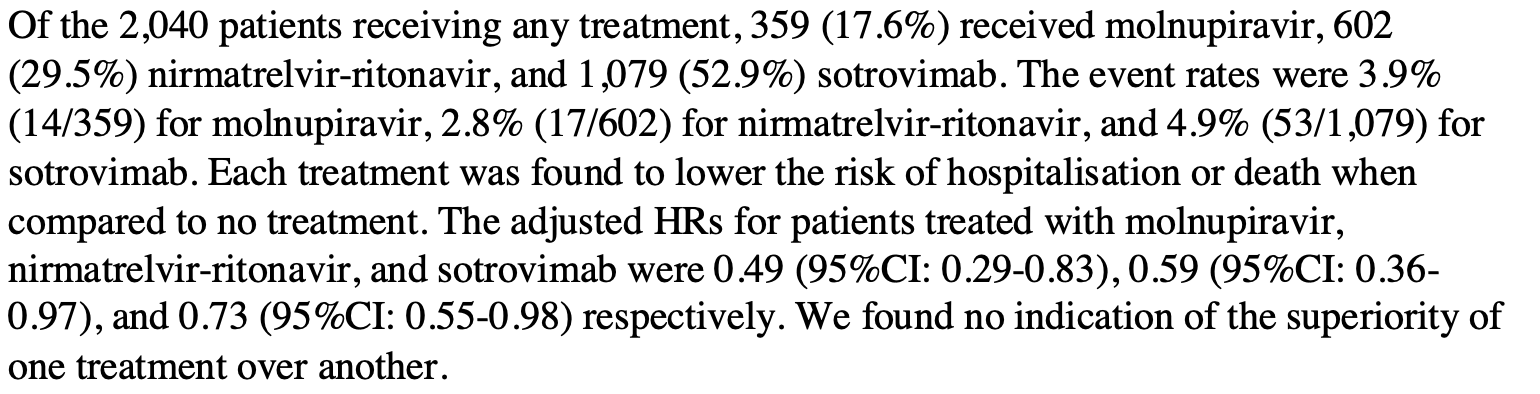
Real-world effectiveness of molnupiravir, nirmatrelvir-ritonavir, and sotrovimab on preventing hospital admission among higher-risk patients with COVID-19 in Wales: A retrospective cohort study
et al., Journal of Infection, doi:10.1016/j.jinf.2023.02.012, Jan 2023
Retrospective high risk outpatients in the UK, showing lower hospitalization/death with paxlovid treatment. Residual confounding is likely with adjustments having no detail on specific comorbidities. Patients with contraindications for paxlovid were not excluded.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments18.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
Study covers molnupiravir, sotrovimab, and paxlovid.
|
risk of death/hospitalization, 41.0% lower, HR 0.59, p = 0.04, treatment 602, control 4,973, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Evans et al., 25 Jan 2023, retrospective, United Kingdom, peer-reviewed, 11 authors, study period 16 December, 2021 - 22 April, 2022.
Contact: andrew.evans@gov.wales, cathy.qi@swansea.ac.uk, j.o.adebayo@swansea.ac.uk, underwoodj7@cardiff.ac.uk, coulsonjm@cardiff.ac.uk, r.bailey@swansea.ac.uk, gareth.john@wales.nhs.uk, edwardsag@cardiff.ac.uk, coopera8@cardiff.ac.uk, r.a.lyons@swansea.ac.uk, a.akbari@swansea.ac.uk.
Real-world effectiveness of molnupiravir, nirmatrelvir-ritonavir, and sotrovimab on preventing hospital admission among higher-risk patients with COVID-19 in Wales: A retrospective cohort study
Journal of Infection, doi:10.1016/j.jinf.2023.02.012
Objective: To compare the effectiveness of molnupiravir, nirmatrelvir-ritonavir, and sotrovimab with no treatment in preventing hospital admission or death in higher-risk patients infected with SARS-CoV-2 in the community. Design: Retrospective cohort study of non-hospitalized adult patients with COVID-19 using the Secure Anonymised Information Linkage (SAIL) Databank. Setting: A real-world cohort study was conducted within the SAIL Databank (a secure trusted research environment containing anonymised, individual, population-scale electronic health record (EHR) data) for the population of Wales, UK. Participants: Adult patients with COVID-19 in the community, at higher risk of hospitalization and death, testing positive for SARS-CoV-2 between 16th December 2021 and 22nd April 2022. Interventions: Molnupiravir, nirmatrelvir-ritonavir, and sotrovimab given in the community by local health boards and the National Antiviral Service in Wales. Main outcome measures: All-cause admission to hospital or death within 28 days of a positive test for SARS-CoV-2. Statistical analysis: Cox proportional hazard model with treatment status (treated/untreated) as a timedependent covariate and adjusted for age, sex, number of comorbidities, Welsh Index of Multiple Deprivation, and vaccination status. Secondary subgroup analyses were by treatment type, number of comorbidities, and before and on or after 20th February 2022, when omicron BA.1 and omicron BA.2 were the dominant subvariants in Wales. Results: Between 16th December 2021 and 22nd April 2022, 7013 higher-risk patients were eligible for inclusion in the study. Of these, 2040 received treatment with molnupiravir (359, 17.6%), nirmatrelvir-ritonavir (602, 29.5%), or sotrovimab (1079, 52.9%). Patients in the treatment group were younger (mean age 53 vs 57 years), had fewer comorbidities, and a higher proportion had received four or more doses of the COVID-19 vaccine (36.3% vs 17.6%). Within 28 days of a positive test, 628 (9.0%) patients were admitted to hospital or died (84 treated and 544 untreated). The primary analysis indicated a lower risk of hospitalization or death at any point within 28 days in treated participants compared to those not receiving treatment. The adjusted hazard rate was 35% (95% CI: 18-49%) lower in treated than untreated participants. There was no indication of the superiority of one treatment over another and no evidence of a reduction in Journal of Infection xxx (xxxx) xxx-xxx
Ethics approval This project uses anonymised individual-level data sources held within the Trusted Research Environment provided by the SAIL Databank at Swansea University, Swansea, UK. All proposals to use SAIL data are subject to review by the independent Information Governance Review Panel (IGRP). This work was approved under proposal number 0911 after careful considerations by IGRP Panel. Access to data is gained through a privacy-protecting safe haven and remote access system referred to as the SAIL Gateway.
CRediT authorship contribution statement All authors were involved in the Conceptualization and design of the study. AE, GJ, AA, CQ, LA and RB contributed to the acquisition of data. CQ, LA and RB carried out the statistical analysis. AE, CQ and LA drafted the original version of the manuscript. All authors contributed to the interpretation of the results and critical revision of the manuscript. All authors approved the final manuscript. AE is the guarantor for this study. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Reporting We used the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) checklist to guide our transparent reporting of our work. A completed STROBE Statement -Checklist of items that should be included in reports of cohort studies is provided.
Appendix A. Supplementary material Supplementary data associated with this article..
References
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med
Butler, Hobbs, Gbinigie, Rahman, Hayward et al., Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet, doi:10.1016/S0140-6736(22)02597-1
Cheng, Reyes, Satram, Birch, Gibbons et al., Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-CoV-2 Delta and Omicron waves in the United States
Cox, Peacock, Harvey, Hughes, Wright, SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies, Nat Rev Microbiol
Ford, Jones, Verplanke, Lyons, Brown, The SAIL databank: building a national architecture for e-health research and evaluation, BMC Health Serv Res
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early remdesivir to prevent progression to severe Covid-19 in outpatients, N Engl J Med
Government, Coronavirus (COVID-19) infection survey (positivity estimates
Government, Weekly COVID-19 treatment counts by therapeutic agent in Wales
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med
Jones, Ford, Jones, Dsilva, Thompson et al., A case study of the secure anonymous information linkage (SAIL) gateway: a privacy protecting remote access system for health related research and evaluation, J Biomed Inform
Koslov, Merck's COVID pill loses its lustre: what that means for the pandemic, Nature, doi:10.1038/d41586-021-03667-0
Lyons, Jones, John, Brooks, Verplanke et al., The SAIL databank: linking multiple health and social care datasets, BMC Med Inform Decis Mak
Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients, Clin Infect Dis
Nhs England, COVID-19 therapeutics (antivirals, neutralising monoclonal antibodies and interleukin 6 inhibitors)
Nhs England, Interim clinical commissioning policy: treatments for non-hospitalised patients with COVID-19
Patel, Yarwood, Levick, Gibbons, Drysdale et al., Characteristics and outcomes of patients with COVID-19 at high-risk of disease progression receiving sotrovimab, oral antivirals or no treatment in England, doi:10.1101/2022.11.28.22282808
Piccicacco, Zeitler, Ing, Montero, Faughn et al., Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the Omicron surge, J Antimicrob Chemother
Radcliffe, Palacios, Azar, Cohen, Malinis, Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge, Am J Transplant
Roche, Ronapreve does not retain neutralising activity against the Omicron variant
Rodgers, Demmler, Dsilva, Lyons, Protecting health data privacy while using residence-based environment and demographic data, Health Place
Rodgers, Lyons, Dsilva, Jones, Brooks et al., Residential anonymous linking fields (RALFs): a novel information infrastructure to study the interaction between the environment and individuals' health, J Public Health
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med
Wu, Carr, Harvey, Mears, Kjaer et al., WHO's therapeutics and COVID-19 living guideline on mAbs needs to be reassessed, Lancet, doi:10.1016/S0140-6736(22)01938-9
Zheng, Green, Tazare, Curtis, Fisher et al., Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform, BMJ
DOI record:
{
"DOI": "10.1016/j.jinf.2023.02.012",
"ISSN": [
"0163-4453"
],
"URL": "http://dx.doi.org/10.1016/j.jinf.2023.02.012",
"alternative-id": [
"S0163445323000828"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Real-world effectiveness of molnupiravir, nirmatrelvir-ritonavir, and sotrovimab on preventing hospital admission among higher-risk patients with COVID-19 in Wales: A retrospective cohort study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jinf.2023.02.012"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "Crown Copyright © 2023 Published by Elsevier Ltd on behalf of The British Infection Association. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Evans",
"given": "Andrew",
"sequence": "first"
},
{
"affiliation": [],
"family": "Qi",
"given": "Cathy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adebayo",
"given": "Jubril Omololu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Underwood",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Coulson",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bailey",
"given": "Rowena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lyons",
"given": "Ronan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Edwards",
"given": "Adrian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cooper",
"given": "Alison",
"sequence": "additional"
},
{
"affiliation": [],
"family": "John",
"given": "Gareth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Akbari",
"given": "Ashley",
"sequence": "additional"
}
],
"container-title": "Journal of Infection",
"container-title-short": "Journal of Infection",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"journalofinfection.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
2,
10
]
],
"date-time": "2023-02-10T10:47:06Z",
"timestamp": 1676026026000
},
"deposited": {
"date-parts": [
[
2023,
2,
24
]
],
"date-time": "2023-02-24T15:41:42Z",
"timestamp": 1677253302000
},
"indexed": {
"date-parts": [
[
2023,
2,
25
]
],
"date-time": "2023-02-25T05:30:36Z",
"timestamp": 1677303036165
},
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2023,
2
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
2,
1
]
],
"date-time": "2023-02-01T00:00:00Z",
"timestamp": 1675209600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0163445323000828?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0163445323000828?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
2
]
]
},
"published-print": {
"date-parts": [
[
2023,
2
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.jinf.2023.02.012_bib1",
"unstructured": "Department of Health and Social Care. Government launches COVID-19 antivirals taskforce to roll out innovative home treatments this autumn; 2021 [cited 2023 Jan 20]. Available from: 〈https://www.gov.uk/government/news/government-launches-covid-19-antivirals-taskforce-to-roll-out-innovative-home-treatments-this-autumn〉."
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2023.02.012_bib2",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2023.02.012_bib3",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2023.02.012_bib4",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early remdesivir to prevent progression to severe Covid-19 in outpatients",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2023.02.012_bib5",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2108163",
"article-title": "REGEN-COV antibody combination and outcomes in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"issue": "23",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2023.02.012_bib6",
"volume": "385",
"year": "2021"
},
{
"article-title": "COVID-19 Genomics UK Consortium,et al., SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies",
"author": "Cox",
"first-page": "1",
"journal-title": "Nat Rev Microbiol",
"key": "10.1016/j.jinf.2023.02.012_bib7",
"year": "2022"
},
{
"key": "10.1016/j.jinf.2023.02.012_bib8",
"unstructured": "Roche. Ronapreve does not retain neutralising activity against the Omicron variant; 2021 [cited 2023 Jan 20] [internet]. Available from: 〈2021216_roche-statement-on-ronapreve-omicron.pdf〉."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib9",
"unstructured": "US Federal Drugs Administration. Coronavirus (COVID-19) update: FDA limits use of certain monoclonal antibodies to treat COVID-19 due to the Omicron variant; 2022 [cited 2023 Jan 20] [internet]. Available from: 〈https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-certain-monoclonal-antibodies-treat-covid-19-due-omicron〉."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib10",
"unstructured": "World Health Organisation. Therapeutics and COVID-19: living guideline; 2022 [cited 2023 Jan 20] [internet]. Available from: 〈https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.5〉."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib11",
"unstructured": "Department of Health and Social Care. Highest-risk patients eligible for new COVID-19 treatments: a guide for patients; 2022 [cited 2023 Jan 20]. Available from: 〈https://www.gov.uk/government/publications/highest-risk-patients-eligible-for-covid-19-treatments-guide-for-patients/highest-risk-patients-eligible-for-new-covid-19-treatments-a-guide-for-patients〉."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib12",
"unstructured": "NHS England. Interim clinical commissioning policy: treatments for non-hospitalised patients with COVID-19; 2022 [cited 2023 Jan 20] [internet]. Available from: 〈C1710-interim-clinical-commissioning-policy-treatments-for-non-hospitalised-patients-with-covid-19-nov-22.pdf〉 (england.nhs.uk)."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib13",
"unstructured": "NHS England. COVID-19 therapeutics (antivirals, neutralising monoclonal antibodies and interleukin 6 inhibitors); 2022 [cited 2023 Jan 20] [internet]. Available from: 〈https://www.england.nhs.uk/statistics/statistical-work-areas/covid-therapeutics-antivirals-and-neutralising-monoclonal-antibodies/〉."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib14",
"unstructured": "Welsh Government. Weekly COVID-19 treatment counts by therapeutic agent in Wales; 2022 [cited 2023 Jan 20] [internet]. Available from: 〈https://statswales.gov.wales/Catalogue/Health-and-Social-Care/coronavirus-covid-19/covid-19-treatments-by-therapeutic-agent/weeklycovidtreatmentcounts-by-therapeuticagent〉."
},
{
"DOI": "10.1186/1472-6963-9-157",
"article-title": "The SAIL databank: building a national architecture for e-health research and evaluation",
"author": "Ford",
"doi-asserted-by": "crossref",
"first-page": "157",
"journal-title": "BMC Health Serv Res",
"key": "10.1016/j.jinf.2023.02.012_bib15",
"volume": "9",
"year": "2009"
},
{
"DOI": "10.1016/j.jbi.2014.01.003",
"article-title": "A case study of the secure anonymous information linkage (SAIL) gateway: a privacy protecting remote access system for health related research and evaluation",
"author": "Jones",
"doi-asserted-by": "crossref",
"first-page": "196",
"issue": "100",
"journal-title": "J Biomed Inform",
"key": "10.1016/j.jinf.2023.02.012_bib16",
"volume": "50",
"year": "2014"
},
{
"DOI": "10.1186/1472-6947-9-3",
"article-title": "The SAIL databank: linking multiple health and social care datasets",
"author": "Lyons",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "BMC Med Inform Decis Mak",
"key": "10.1016/j.jinf.2023.02.012_bib17",
"volume": "9",
"year": "2009"
},
{
"DOI": "10.1016/j.healthplace.2011.09.006",
"article-title": "Protecting health data privacy while using residence-based environment and demographic data",
"author": "Rodgers",
"doi-asserted-by": "crossref",
"first-page": "209",
"issue": "2",
"journal-title": "Health Place",
"key": "10.1016/j.jinf.2023.02.012_bib18",
"volume": "18",
"year": "2012"
},
{
"DOI": "10.1093/pubmed/fdp041",
"article-title": "Residential anonymous linking fields (RALFs): a novel information infrastructure to study the interaction between the environment and individuals’ health",
"author": "Rodgers",
"doi-asserted-by": "crossref",
"first-page": "582",
"issue": "4",
"journal-title": "J Public Health",
"key": "10.1016/j.jinf.2023.02.012_bib19",
"volume": "31",
"year": "2009"
},
{
"key": "10.1016/j.jinf.2023.02.012_bib20",
"unstructured": "Welsh Government. Coronavirus (COVID-19) infection survey (positivity estimates): 27 March to 2 April 2022; 2022 [cited 2023 Jan 20] [internet]. Available from: 〈https://www.gov.wales/coronavirus-covid-19-infection-survey-positivity-estimates-27-march-2-april-2022-html〉."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib21",
"unstructured": "Welsh Medicines Information Centre. National Antiviral Service Cymru (NAVS) – information for members of the public; 2022 [cited 2023 Jan 20]. Available from: 〈https://www.wmic.wales.nhs.uk/navs-cymru/#:~:text=%E2%80%93%20Information%20for%20Members%20of%20the,not%20been%20admitted%20to%20hospital〉."
},
{
"article-title": "Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients",
"author": "Najjar-Debbiny",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jinf.2023.02.012_bib22",
"year": "2022"
},
{
"DOI": "10.1101/2022.09.07.22279497",
"doi-asserted-by": "crossref",
"key": "10.1016/j.jinf.2023.02.012_bib23",
"unstructured": "Cheng M, Reyes C, Satram S, H. Birch, D.C. Gibbons, M. Drysdale, et al. Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-CoV-2 Delta and Omicron waves in the United States. [Preprint]. medRxiv 2022.09.07.22279497."
},
{
"DOI": "10.1093/jac/dkac256",
"article-title": "Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the Omicron surge",
"author": "Piccicacco",
"doi-asserted-by": "crossref",
"first-page": "2693",
"issue": "10",
"journal-title": "J Antimicrob Chemother",
"key": "10.1016/j.jinf.2023.02.012_bib24",
"volume": "77",
"year": "2022"
},
{
"article-title": "Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial",
"author": "Butler",
"journal-title": "Lancet",
"key": "10.1016/j.jinf.2023.02.012_bib25",
"year": "2022"
},
{
"article-title": "Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform",
"author": "Zheng",
"journal-title": "BMJ",
"key": "10.1016/j.jinf.2023.02.012_bib26",
"volume": "379",
"year": "2022"
},
{
"article-title": "Characteristics and outcomes of patients with COVID-19 at high-risk of disease progression receiving sotrovimab, oral antivirals or no treatment in England",
"author": "Patel",
"journal-title": "medRxiv",
"key": "10.1016/j.jinf.2023.02.012_bib27",
"year": "2022"
},
{
"DOI": "10.1111/ajt.17098",
"article-title": "Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge",
"author": "Radcliffe",
"doi-asserted-by": "crossref",
"first-page": "2458",
"journal-title": "Am J Transplant",
"key": "10.1016/j.jinf.2023.02.012_bib28",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)01938-9",
"article-title": "WHO’s therapeutics and COVID-19 living guideline on mAbs needs to be reassessed",
"author": "Wu",
"doi-asserted-by": "crossref",
"journal-title": "Lancet",
"key": "10.1016/j.jinf.2023.02.012_bib29",
"year": "2022"
},
{
"DOI": "10.1038/d41586-021-03667-0",
"article-title": "Merck’s COVID pill loses its lustre: what that means for the pandemic",
"author": "Koslov",
"doi-asserted-by": "crossref",
"journal-title": "Nature",
"key": "10.1016/j.jinf.2023.02.012_bib30",
"year": "2021"
},
{
"key": "10.1016/j.jinf.2023.02.012_bib31",
"unstructured": "Office for National Statistics. Coronavirus (COVID-19) latest insights: hospitals; 2023 [cited 2023 Jan 20] [internet]. Available from: 〈https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/hospitals〉."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib32",
"unstructured": "The National Institute for Health and Care Excellence. Draft guidance consultation: therapeutics for people with COVID-19; 2022 [cited 2023 Jan 20] [internet]. Available from: 〈https://www.nice.org.uk/guidance/gid-ta10936/documents/129〉."
},
{
"key": "10.1016/j.jinf.2023.02.012_bib33",
"unstructured": "The Institute for Clinical and Economic Review. Report at a glance: COVID-19; 2022 [cited 2023 Jan 20]. Available from: 〈https://icer.org/wp-content/uploads/2022/05/COVID-19-RAAG_10May2022.pdf〉."
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0163445323000828"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Real-world effectiveness of molnupiravir, nirmatrelvir-ritonavir, and sotrovimab on preventing hospital admission among higher-risk patients with COVID-19 in Wales: A retrospective cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}