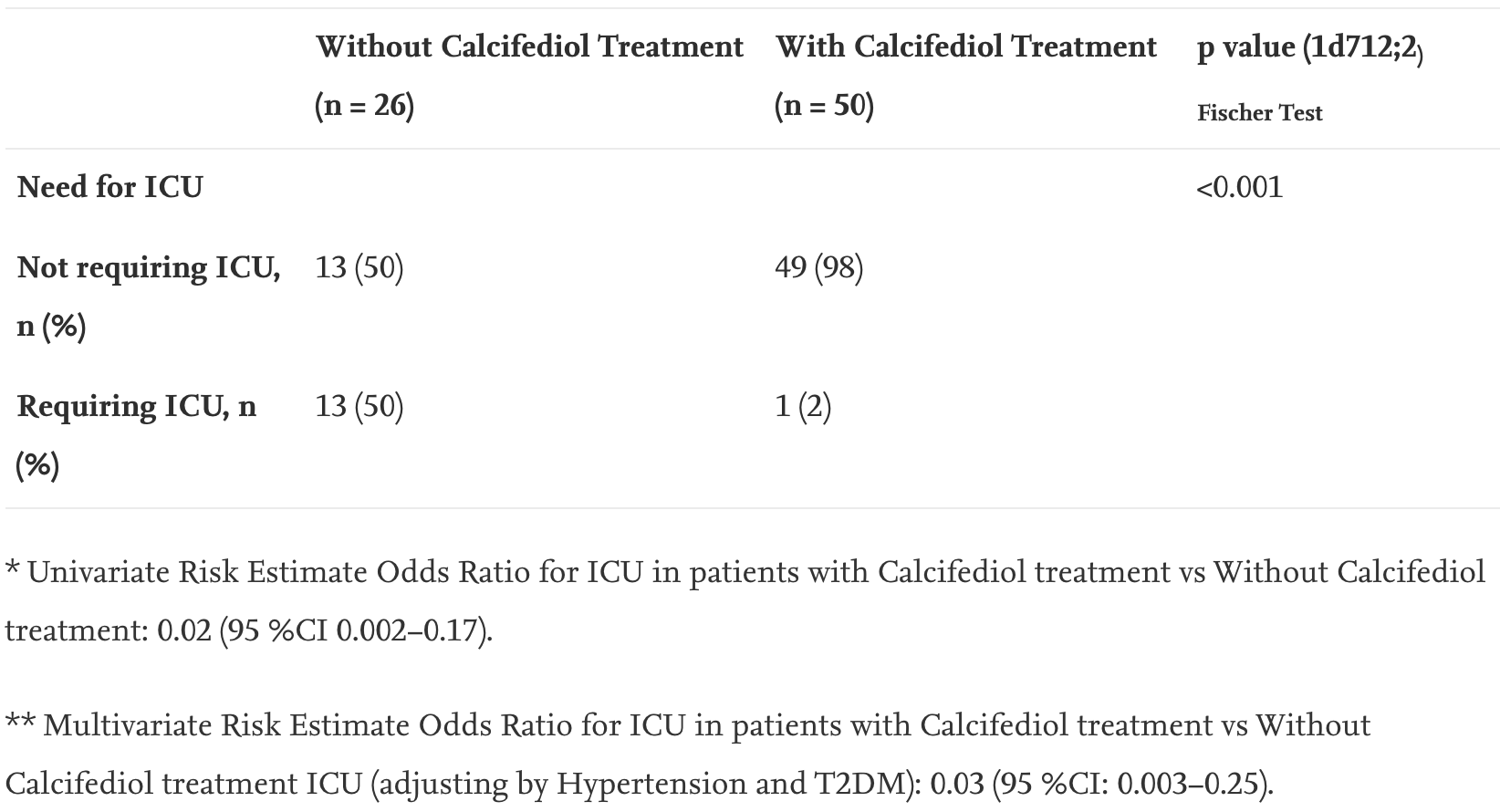
Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study
et al., Journal of Steroid Biochemistry and Molecular Biology, 203, October 2020, doi:10.1016/j.jsbmb.2020.105751, COVIDIOL, NCT04366908, Aug 2020
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT on calcifediol (25-hydroxyvitamin D) treatment for hospitalized COVID-19 patients showing significantly reduced intensive care unit admissions. All patients received standard care including HCQ+AZ. For additional analysis see Jungreis et al.1.
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 45% [34‑54%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
This is the 1st of 40 COVID-19 RCTs for vitamin D, which collectively show efficacy with p=0.0000001.
This is the 3rd of 135 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
|
risk of death, 85.4% lower, RR 0.15, p = 0.11, treatment 0 of 50 (0.0%), control 2 of 26 (7.7%), NNT 13, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of ICU admission, 94.2% lower, RR 0.06, p = 0.008, treatment 1 of 50 (2.0%), control 13 of 26 (50.0%), NNT 2.1, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Entrenas Castillo et al., 29 Aug 2020, Randomized Controlled Trial, Spain, peer-reviewed, 7 authors, study period May 2020 - June 2020, dosage calcifediol 0.5mg day 1, 0.27mg day 3, 0.27mg day 7, and then weekly until discharge or ICU admission, trial NCT04366908 (history) (COVIDIOL).
Calcifediol or Corticosteroids in the Treatment of COVID-19: An Observational Study
Nutrients, doi:10.3390/nu16121910
Medical treatment of coronavirus 19 disease (COVID-19) is a therapeutic challenge. The available data strongly suggest that calcifediol treatment may reduce the severity of COVID-19, and corticosteroids are the treatment of choice worldwide for severe COVID-19. Both have a very similar action profile, and their combined use in patients may modify the contribution of each administered compound. Objective: To evaluate how treatment with calcifediol and/or corticosteroids in medical practice modified the need for ICU admission, death, or poor prognosis of patients hospitalized with COVID-19 during the first outbreaks. Design, patients and setting: A retrospective observational cohort study of patients admitted for COVID-19 to the Pneumology Unit of the Hospital Universitario Reina Sofía (Córdoba, Spain). Interventions: Patients were treated with calcifediol or/and corticosteroids with the best available therapy and standard care, according to clinical practice guidelines. Measurements: Admission to the intensive care unit (ICU) or death during hospitalization and poor prognosis. Results: Seven hundred and twenty-eight patients were included. According to the treatment received, they were included in four groups: calcifediol (n = 68), glucocorticoids (n = 112), both (n = 510), or neither (n = 38). Of the 578 patients treated with calcifediol, 88 were admitted to the ICU (15%), while of the 150 not treated with calcifediol, 39 required ICU admission (26%) (p < 0.01). Among the patients taking calcifediol without glucocorticoids, only 4 of 68 (5.8%) required ICU admission, compared to 84 of 510 (16.5%) treated with both (p = 0.022). Of the 595 patients who had a good prognosis, 568 (82.01%) had received treatment with calcifediol versus the 133 patients with a poor prognosis, of whom 90 (67.66%) had received calcifediol (p < 0.001). This difference was not found for corticosteroids. Interpretation: The treatment of choice for hospitalized patients with moderate or mild COVID-19 could be calcifediol, not administering corticosteroids, until the natural history of the disease reaches a stage of hyperinflammation.
Funding: This study was funded by an Intramural Grant of Clinical trial funds (ECL 026) from FIBICO (Fundación del Instituto Maimónides de Investigación Biomédica de Córdoba).
Institutional Review Board Statement: The study was approved by the Biomedical Research Ethics Committee of the Hospital Universitario Reina Sofía de Córdoba (committee reference number 5291)
Conflicts of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. The funder (FIBICO) had no role in the design of the study; in the collection, analyses or interpretation of the data; in the writing of the manuscript or in the decision to publish the results.
References
Alcala-Diaz, Limia-Perez, Gomez-Huelgas, Martin-Escalante, Cortes-Rodriguez et al., Calcifediol treatment and hospital mortality due to COVID-19: A cohort study, Nutrients, doi:10.3390/nu13061760
Altmann, Whettlock, Liu, Arachchillage, Boyton, The immunology of long covid, Nat. Rev. Immunol, doi:10.1038/S41577-023-00904-7
Blanco-Melo, Nilsson-Payant, Liu, Uhl, Hoagland et al., Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19, Cell, doi:10.1016/J.CELL.2020.04.026
Bouillon, Quesada-Gomez, Vitamin D Endocrine System and COVID-19, JBMR Plus, doi:10.1002/JBM4.10576
Caiazzo, Rezig, Bruzzese, Ialenti, Cicala et al., Systemic administration of glucocorticoids, cardiovascular complications and mortality in patients hospitalised with COVID-19, SARS, MERS or influenza: A systematic review and meta-analysis of randomised trials, Pharmacol. Res, doi:10.1016/J.PHRS.2021.106053
Castillo, Entrenas Costa, Vaquero Barrios, Alcalá Díaz, López Miranda et al., Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2020.105751
Castillo-Peinado, Calderón-Santiago, Sánchez-Cano, Quesada-Gómez, Bouillon et al., Determination of vitamin D3 conjugated metabolites: A complementary view on hydroxylated metabolites, Analyst, doi:10.1039/D2AN01982E
Dagens, Sigfrid, Cai, Lipworth, Cheung et al., Scope, quality, and inclusivity of clinical guidelines produced early in the COVID-19 pandemic: Rapid review, BMJ, doi:10.1136/BMJ.M1936
Efird, Anderson, Jindal, Redding, Thompson et al., The interaction of Vitamin D and corticosteroids: A mortality analysis of 26,508 veterans who tested positive for SARS-CoV-2, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph19010447
Efird, Anderson, Jindal, Suzuki, Interaction of Vitamin D and Corticosteroid Use in Hospitalized COVID-19 Patients: A Potential Explanation for Inconsistent Findings in the Literature, Curr. Pharm. Des, doi:10.2174/1381612828666220418132847
Ghazy, Almaghraby, Shaaban, Kamal, Beshir et al., A systematic review and meta-analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID-19 treatment, Sci. Rep, doi:10.1038/S41598-020-77748-X
Gupta, Madhavan, Sehgal, Nair, Mahajan et al., Extrapulmonary manifestations of COVID-19, Nat. Med, doi:10.1038/S41591-020-0968-3
Horby, Lim, Emberson, Mafham, Bell et al., Dexamethasone in Hospitalized Patients with COVID-19, N. Engl. J. Med, doi:10.1056/nejmoa2021436
Jenkinson, Desai, Mcleod, Wolf Mueller, Hewison et al., Circulating Conjugated and Unconjugated Vitamin D Metabolite Measurements by Liquid Chromatography Mass Spectrometry, J. Clin. Endocrinol. Metab, doi:10.1210/CLINEM/DGAB708
Kato, Nishiyama, Nishimura, Noda, Okabe et al., Drug repurposing for the treatment of COVID-19, J. Pharmacol. Sci, doi:10.1016/J.JPHS.2022.04.007
Kaufman, Niles, Kroll, Bi, Holick, SARS-CoV-2 positivity rates associated with circulating 25hydroxyvitamin D levels, PLoS ONE, doi:10.1371/JOURNAL.PONE.0239252
Ketha, Thacher, Oberhelman, Fischer, Singh et al., Comparison of the effect of daily versus bolus dose maternal vitamin D3 supplementation on the 24,25-dihydroxyvitamin D3 to 25-hydroxyvitamin D3 ratio, Bone, doi:10.1016/J.BONE.2018.02.024
Kulkarni, Gunnarsson, Yi, Gudmundsdottir, Sigurjonsson et al., Glucocorticoid dexamethasone down-regulates basal and vitamin D3 induced cathelicidin expression in human monocytes and bronchial epithelial cell line, Immunobiology, doi:10.1016/J.IMBIO.2015.09.001
Liu, Zhang, Dong, Li, Xu et al., Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome, J. Clin. Investig, doi:10.1172/JCI140617
Loucera, Peña-Chilet, Esteban-Medina, Muñoyerro-Muñiz, Villegas et al., Real world evidence of calcifediol or vitamin D prescription and mortality rate of COVID-19 in a retrospective cohort of hospitalized Andalusian patients, Sci. Rep
Lugg, Thickett, The role of vitamin D in COVID-19, doi:10.1016/B978-0-323-91338-6.00049-5
Luis, Talía, David, Laura, María et al., Documento Técnico Manejo Clínico del COVID-19
Maghbooli, Sahraian, Jamalimoghadamsiahkali, Asadi, Zarei et al., Treatment with 25-Hydroxyvitamin D3 (Calcifediol) Is Associated with a Reduction in the Blood Neutrophil-to-Lymphocyte Ratio Marker of Disease Severity in Hospitalized Patients with COVID-19: A Pilot Multicenter, Randomized, Placebo-Controlled, Double-Bli, Endocr. Pract, doi:10.1016/j.eprac.2021.09.016
Marcellini, Swieboda, Guedán, Farrow, Casolari et al., Glucocorticoids impair type I IFN signalling and enhance rhinovirus replication, Eur. J. Pharmacol, doi:10.1016/J.EJPHAR.2020.173839
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., Consider cytokine storm syndromes and immunosuppression, Lancet, doi:10.1016/S0140-6736(20)30628-0
Meltzer, Best, Zhang, Vokes, Arora et al., Association of Vitamin D Status and Other Clinical Characteristics with COVID-19 Test Results, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2020.19722
Merad, Blish, Sallusto, Iwasaki, The immunology and immunopathology of COVID-19, Science, doi:10.1126/SCIENCE.ABM8108
Mingiano, Picchioni, Cavati, Pirrotta, Calabrese et al., Vitamin D Deficiency in COVID-19 Patients and Role of Calcifediol Supplementation, Nutrients, doi:10.3390/NU15153392
Murakami, Hayden, Hills, Al-Samkari, Casey et al., Therapeutic advances in COVID-19, Nat. Rev. Nephrol, doi:10.1038/S41581-022-00642-4
Nogues, Ovejero, Pineda-Moncusí, Bouillon, Arenas et al., Calcifediol treatment and COVID-19-Related outcomes, J. Clin. Endocrinol. Metab, doi:10.1210/clinem/dgab405
Quesada-Gomez, Bouillon, Is calcifediol better than cholecalciferol for vitamin D supplementation? 25OHD 25-Hydroxyvitamin D 3 and 25-hydroxyvitamin D 2 combined in plasma Vitamin D Vitamin D 3 or D 2 RCT Randomized controlled trial, Osteoporos. Int, doi:10.1016/0032-3861(91)90593-8
Quesada-Gomez, Entrenas-Castillo, Bouillon, Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS-CoV-2 infections: Revised Ms SBMB 2020_166, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2020.105719
Quesada-Gomez, Lopez-Miranda, Entrenas-Castillo, Casado-Díaz, Nogues et al., Vitamin D Endocrine System and COVID-19: Treatment with Calcifediol, Nutrients, doi:10.3390/NU14132716
Ranabhat, Jakovljevic, Kim, Simkhada, COVID-19 Pandemic: An Opportunity for Universal Health Coverage, Front. Public Health, doi:10.3389/FPUBH.2021.673542
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area, JAMA J. Am. Med. Assoc, doi:10.1001/jama.2020.6775
Rysz, Al-Saadi, Sjöström, Farm, Campoccia Jalde et al., COVID-19 pathophysiology may be driven by an imbalance in the renin-angiotensin-aldosterone system, Nat. Commun, doi:10.1038/S41467-021-22713-Z
Sarzani, Spannella, Giulietti, Di Pentima, Giordano et al., Possible harm from glucocorticoid drugs misuse in the early phase of SARS-CoV-2 infection: A narrative review of the evidence, Intern. Emerg. Med, doi:10.1007/S11739-021-02860-3
Shimba, Ikuta, Glucocorticoids Regulate Circadian Rhythm of Innate and Adaptive Immunity, Front. Immunol, doi:10.3389/FIMMU.2020.02143
Smolders, Van Den Ouweland, Geven, Pickkers, Kox, Letter to the Editor: Vitamin D deficiency in COVID-19: Mixing up cause and consequence, Metab. Clin. Experimental, doi:10.1016/J.METABOL.2020.154434
Spiezia, Boscolo, Poletto, Cerruti, Tiberio et al., COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure, Thromb. Haemost, doi:10.1055/S-0040-1710018
Vandewalle, Luypaert, De Bosscher, Libert, Therapeutic Mechanisms of Glucocorticoids, Trends Endocrinol. Metab, doi:10.1016/J.TEM.2017.10.010
Wang, Joshi, Leopold, Jackson, Christensen et al., Association of vitamin D deficiency with COVID-19 infection severity: Systematic review and meta-analysis, Clin. Endocrinol, doi:10.1111/CEN.14540
Wang, Yang, Chen, Guo, Liu et al., The proportion and effect of corticosteroid therapy in patients with COVID-19 infection: A systematic review and meta-analysis, PLoS ONE, doi:10.1371/JOURNAL.PONE.0249481
Wang, Yang, Li, Huang, Jiang et al., Specific cytokines in the inflammatory cytokine storm of patients with COVID-19-associated acute respiratory distress syndrome and extrapulmonary multiple-organ dysfunction, Virol. J, doi:10.1186/S12985-021-01588-Y
Wiersinga, Rhodes, Cheng, Peacock, Prescott et al., Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review, JAMA, doi:10.1001/JAMA.2020.12839
Young, Clyne, Chapman, Endocrine aspects of ACE2 regulation: RAAS, steroid hormones and SARS-CoV-2, J. Endocrinol, doi:10.1530/JOE-20-0260
Zheng, Yang, Hu, Li, Wang et al., Vitamin D attenuates lung injury via stimulating epithelial repair, reducing epithelial cell apoptosis and inhibits TGF-β induced epithelial to mesenchymal transition, Biochem. Pharmacol, doi:10.1016/j.bcp.2020.113955
DOI record:
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"ISSN": [
"0960-0760"
],
"URL": "http://dx.doi.org/10.1016/j.jsbmb.2020.105751",
"alternative-id": [
"S0960076020302764"
],
"article-number": "105751",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "“Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study”"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Journal of Steroid Biochemistry and Molecular Biology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jsbmb.2020.105751"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 Published by Elsevier Ltd."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-2264-3456",
"affiliation": [],
"authenticated-orcid": false,
"family": "Entrenas Castillo",
"given": "Marta",
"sequence": "first"
},
{
"affiliation": [],
"family": "Entrenas Costa",
"given": "Luis Manuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vaquero Barrios",
"given": "José Manuel",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4572-3611",
"affiliation": [],
"authenticated-orcid": false,
"family": "Alcalá Díaz",
"given": "Juan Francisco",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8844-0718",
"affiliation": [],
"authenticated-orcid": false,
"family": "López Miranda",
"given": "José",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bouillon",
"given": "Roger",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Quesada Gomez",
"given": "José Manuel",
"sequence": "additional"
}
],
"container-title": "The Journal of Steroid Biochemistry and Molecular Biology",
"container-title-short": "The Journal of Steroid Biochemistry and Molecular Biology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
8,
29
]
],
"date-time": "2020-08-29T15:13:41Z",
"timestamp": 1598714021000
},
"deposited": {
"date-parts": [
[
2024,
1,
27
]
],
"date-time": "2024-01-27T06:00:47Z",
"timestamp": 1706335247000
},
"indexed": {
"date-parts": [
[
2024,
4,
8
]
],
"date-time": "2024-04-08T08:30:48Z",
"timestamp": 1712565048616
},
"is-referenced-by-count": 486,
"issued": {
"date-parts": [
[
2020,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
1
]
],
"date-time": "2020-10-01T00:00:00Z",
"timestamp": 1601510400000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
1
]
],
"date-time": "2020-10-01T00:00:00Z",
"timestamp": 1601510400000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
1
]
],
"date-time": "2020-10-01T00:00:00Z",
"timestamp": 1601510400000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
1
]
],
"date-time": "2020-10-01T00:00:00Z",
"timestamp": 1601510400000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
1
]
],
"date-time": "2020-10-01T00:00:00Z",
"timestamp": 1601510400000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
1
]
],
"date-time": "2020-10-01T00:00:00Z",
"timestamp": 1601510400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0960076020302764?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0960076020302764?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "105751",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2020,
10
]
]
},
"published-print": {
"date-parts": [
[
2020,
10
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1038/s41586-020-2012-7",
"article-title": "A pneumonia outbreak associated with a new coronavirus of probable bat origin",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "270",
"journal-title": "Nature",
"key": "10.1016/j.jsbmb.2020.105751_bib0005",
"volume": "579",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Lancet",
"key": "10.1016/j.jsbmb.2020.105751_bib0010",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"article-title": "Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "507",
"journal-title": "Lancet",
"key": "10.1016/j.jsbmb.2020.105751_bib0015",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jama.2017.21907",
"article-title": "Acute respiratory distress syndrome",
"author": "Fan",
"doi-asserted-by": "crossref",
"first-page": "698",
"journal-title": "JAMA",
"key": "10.1016/j.jsbmb.2020.105751_bib0020",
"volume": "319",
"year": "2018"
},
{
"article-title": "Acute respiratory distress syndrome: the Berlin definition",
"author": "Ranieri",
"first-page": "2526",
"journal-title": "JAMA - J. Am. Med. Assoc.",
"key": "10.1016/j.jsbmb.2020.105751_bib0025",
"volume": "307",
"year": "2012"
},
{
"DOI": "10.1001/jamainternmed.2020.0994",
"article-title": "Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China",
"author": "Wu",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Intern. Med.",
"key": "10.1016/j.jsbmb.2020.105751_bib0030",
"year": "2020"
},
{
"DOI": "10.1001/jama.2016.0291",
"article-title": "Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries",
"author": "Bellani",
"doi-asserted-by": "crossref",
"first-page": "788",
"journal-title": "JAMA - J. Am. Med. Assoc.",
"key": "10.1016/j.jsbmb.2020.105751_bib0035",
"volume": "315",
"year": "2016"
},
{
"DOI": "10.1016/S2213-2600(20)30076-X",
"article-title": "Pathological findings of COVID-19 associated with acute respiratory distress syndrome",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "420",
"journal-title": "Lancet Respir. Med.",
"key": "10.1016/j.jsbmb.2020.105751_bib0040",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.8115",
"article-title": "Randomized clinical trials and COVID-19: managing expectations",
"author": "Bauchner",
"doi-asserted-by": "crossref",
"journal-title": "JAMA - J. Am. Med. Assoc.",
"key": "10.1016/j.jsbmb.2020.105751_bib0045",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.8297",
"article-title": "Estimated demand for US hospital inpatient and intensive care unit beds for patients with COVID-19 based on comparisons with Wuhan and Guangzhou, China",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "e208297",
"journal-title": "JAMA Netw. Open.",
"key": "10.1016/j.jsbmb.2020.105751_bib0050",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1016/j.jsbmb.2020.105719",
"article-title": "Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS-CoV-2 infections: revised Ms SBMB 2020_166",
"author": "Quesada-Gomez",
"doi-asserted-by": "crossref",
"journal-title": "J. Steroid Biochem. Mol. Biol.",
"key": "10.1016/j.jsbmb.2020.105751_bib0055",
"volume": "202",
"year": "2020"
},
{
"DOI": "10.3892/mmr.2015.4685",
"article-title": "Vitamin D/VDR signaling attenuates lipopolysaccharide-induced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier",
"author": "Shi",
"doi-asserted-by": "crossref",
"first-page": "1186",
"journal-title": "Mol. Med. Rep.",
"key": "10.1016/j.jsbmb.2020.105751_bib0060",
"volume": "13",
"year": "2016"
},
{
"DOI": "10.3892/mmr.2015.4685",
"article-title": "Vitamin D/VDR signaling attenuates lipopolysaccharide-induced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier",
"author": "Shi",
"doi-asserted-by": "crossref",
"first-page": "1186",
"journal-title": "Mol. Med. Rep.",
"key": "10.1016/j.jsbmb.2020.105751_bib0065",
"volume": "13",
"year": "2016"
},
{
"DOI": "10.1210/me.2013-1146",
"article-title": "VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system",
"author": "Kong",
"doi-asserted-by": "crossref",
"first-page": "2116",
"journal-title": "Mol. Endocrinol.",
"key": "10.1016/j.jsbmb.2020.105751_bib0070",
"volume": "27",
"year": "2013"
},
{
"DOI": "10.1016/j.bcp.2020.113955",
"article-title": "Vitamin D attenuates lung injury via stimulating epithelial repair, reducing epithelial cell apoptosis and inhibits TGF-β induced epithelial to mesenchymal transition",
"author": "Zheng",
"doi-asserted-by": "crossref",
"journal-title": "Biochem. Pharmacol.",
"key": "10.1016/j.jsbmb.2020.105751_bib0075",
"volume": "177",
"year": "2020"
},
{
"DOI": "10.1096/fj.15-272872",
"article-title": "Vitamin D modulates tissue factor and protease-activated receptor 2 expression in vascular smooth muscle cells",
"author": "Martinez-Moreno",
"doi-asserted-by": "crossref",
"first-page": "1367",
"journal-title": "FASEB J.",
"key": "10.1016/j.jsbmb.2020.105751_bib0080",
"volume": "30",
"year": "2016"
},
{
"article-title": "Vitamin D. And inflammation: potential implications for severity of Covid-19",
"author": "Laird",
"journal-title": "Ir. Med. J.",
"key": "10.1016/j.jsbmb.2020.105751_bib0085",
"volume": "113",
"year": "2020"
},
{
"DOI": "10.1007/s40520-020-01570-8",
"article-title": "The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality",
"author": "Ilie",
"doi-asserted-by": "crossref",
"first-page": "1195",
"journal-title": "Aging Clin. Exp. Res.",
"key": "10.1016/j.jsbmb.2020.105751_bib0090",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.3390/nu12051359",
"article-title": "25-hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2",
"author": "D’avolio",
"doi-asserted-by": "crossref",
"journal-title": "Nutrients",
"key": "10.1016/j.jsbmb.2020.105751_bib0095",
"volume": "12",
"year": "2020"
},
{
"article-title": "Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalised with COVID-19 are associated with greater disease severity",
"author": "Panagiotou",
"journal-title": "Clin. Endocrinol. (Oxf).",
"key": "10.1016/j.jsbmb.2020.105751_bib0100",
"year": "2020"
},
{
"DOI": "10.1016/j.clinbiochem.2008.02.003",
"article-title": "Inappropriate serum levels of retinol, α-tocopherol, 25 hydroxyvitamin D3 and 24,25 dihydroxyvitamin D3 levels in healthy Spanish adults: simultaneous assessment by HPLC",
"author": "Mata-Granados",
"doi-asserted-by": "crossref",
"first-page": "676",
"journal-title": "Clin. Biochem.",
"key": "10.1016/j.jsbmb.2020.105751_bib0105",
"volume": "41",
"year": "2008"
},
{
"DOI": "10.1016/j.jsbmb.2010.03.078",
"article-title": "Evaluation of vitamin D endocrine system (VDES) status and response to treatment of patients in intensive care units (ICUs) using an on-line SPE-LC-MS/MS method",
"author": "Mata-Granados",
"doi-asserted-by": "crossref",
"first-page": "452",
"journal-title": "J. Steroid Biochem. Mol. Biol.",
"key": "10.1016/j.jsbmb.2020.105751_bib0110",
"volume": "121",
"year": "2010"
},
{
"key": "10.1016/j.jsbmb.2020.105751_bib0115",
"series-title": "Gobierno De España, Ministerio De Sanidad, Consumo Y Bienestar Social - Documentos Técnicos Para Profesionales - Coronavirus",
"year": "2020"
},
{
"key": "10.1016/j.jsbmb.2020.105751_bib0120",
"unstructured": "Tratamientos disponibles sujetos a condiciones especiales de acceso para el manejo de la infección respiratoria por SARS-CoV-2 - Agencia Española de Medicamentos y Productos Sanitarios, (n.d.). https://www.aemps.gob.es/la-aemps/ultima-informacion-de-la-aemps-acerca-del-covid-19/tratamientos-disponibles-para-el-manejo-de-la-infeccion-respiratoria-por-sars-cov-2/?lang=en (accessed June 22, 2020)."
},
{
"DOI": "10.1136/thorax.58.5.377",
"article-title": "Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study",
"author": "Lim",
"doi-asserted-by": "crossref",
"first-page": "377",
"journal-title": "Thorax",
"key": "10.1016/j.jsbmb.2020.105751_bib0125",
"volume": "58",
"year": "2003"
},
{
"key": "10.1016/j.jsbmb.2020.105751_bib0130",
"unstructured": "In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) - PubMed, (n.d.). https://pubmed.ncbi.nlm.nih.gov/32150618/ (accessed June 23, 2020)."
},
{
"DOI": "10.1086/511159",
"article-title": "Infectious diseases society of America/American thoracic society consensus guidelines on the management of community-acquired pneumonia in adults",
"author": "Mandell",
"doi-asserted-by": "crossref",
"first-page": "S27",
"journal-title": "Clin. Infect. Dis.",
"key": "10.1016/j.jsbmb.2020.105751_bib0135",
"volume": "44",
"year": "2007"
},
{
"DOI": "10.1016/j.jclinepi.2015.04.014",
"article-title": "A simple formula for the calculation of sample size in pilot studies",
"author": "Viechtbauer",
"doi-asserted-by": "crossref",
"first-page": "1375",
"journal-title": "J. Clin. Epidemiol.",
"key": "10.1016/j.jsbmb.2020.105751_bib0140",
"volume": "68",
"year": "2015"
},
{
"DOI": "10.1007/s00223-011-9513-1",
"article-title": "Metabolic changes following 500 μg monthly administration of calcidiol: a study in normal females",
"author": "Russo",
"doi-asserted-by": "crossref",
"first-page": "252",
"journal-title": "Calcif. Tissue Int.",
"key": "10.1016/j.jsbmb.2020.105751_bib0145",
"volume": "89",
"year": "2011"
},
{
"key": "10.1016/j.jsbmb.2020.105751_bib0150",
"unstructured": "Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases, (n.d.). https://www.who.int/publications/i/item/10665-331501 (accessed June 23, 2020)."
},
{
"DOI": "10.1186/1741-7015-8-18",
"article-title": "CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials",
"author": "Schulz",
"doi-asserted-by": "crossref",
"journal-title": "BMC Med.",
"key": "10.1016/j.jsbmb.2020.105751_bib0155",
"volume": "8",
"year": "2010"
},
{
"DOI": "10.1056/NEJMoa2012410",
"article-title": "Observational study of hydroxychloroquine in hospitalized patients with COVID-19",
"author": "Geleris",
"doi-asserted-by": "crossref",
"first-page": "2411",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.jsbmb.2020.105751_bib0160",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1210/er.2018-00126",
"article-title": "Skeletal and extraskeletal actions of vitamin d: current evidence and outstanding questions",
"author": "Bouillon",
"doi-asserted-by": "crossref",
"first-page": "1109",
"journal-title": "Endocr. Rev.",
"key": "10.1016/j.jsbmb.2020.105751_bib0165",
"volume": "40",
"year": "2019"
},
{
"DOI": "10.4049/jimmunol.181.10.7090",
"article-title": "Respiratory epithelial cells convert inactive vitamin d to its active form: potential effects on host defense",
"author": "Hansdottir",
"doi-asserted-by": "crossref",
"first-page": "7090",
"journal-title": "J. Immunol.",
"key": "10.1016/j.jsbmb.2020.105751_bib0170",
"volume": "181",
"year": "2008"
},
{
"DOI": "10.1371/journal.pone.0215383",
"article-title": "25(OH)D 3 and 1.25(OH) 2 D 3 inhibits TNF-α expression in human monocyte derived macrophages",
"author": "Rafique",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/j.jsbmb.2020.105751_bib0175",
"volume": "14",
"year": "2019"
},
{
"DOI": "10.1371/journal.pone.0090301",
"article-title": "Both 25-hydroxyvitamin-D3 and 1,25-dihydroxyvitamin- D3 reduces inflammatory response in human periodontal ligament cells",
"author": "Andrukhov",
"doi-asserted-by": "crossref",
"first-page": "e90301",
"journal-title": "PLoS One",
"key": "10.1016/j.jsbmb.2020.105751_bib0180",
"volume": "9",
"year": "2014"
},
{
"DOI": "10.1002/oby.22831",
"article-title": "High prevalence of obesity in severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation",
"author": "Simonnet",
"doi-asserted-by": "crossref",
"first-page": "1195",
"journal-title": "Obesity.",
"key": "10.1016/j.jsbmb.2020.105751_bib0185",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1016/S2213-8587(20)30268-0",
"article-title": "Vitamin D for COVID-19: a case to answer?",
"author": "Martineau",
"doi-asserted-by": "crossref",
"journal-title": "Lancet Diabetes Endocrinol.",
"key": "10.1016/j.jsbmb.2020.105751_bib0190",
"year": "2020"
},
{
"DOI": "10.1111/nure.12090",
"article-title": "Guidelines for optimizing design and analysis of clinical studies of nutrient effects",
"author": "Heaney",
"doi-asserted-by": "crossref",
"first-page": "48",
"journal-title": "Nutr. Rev.",
"key": "10.1016/j.jsbmb.2020.105751_bib0195",
"volume": "72",
"year": "2014"
},
{
"DOI": "10.1016/j.jsbmb.2017.08.009",
"article-title": "Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations",
"author": "Grant",
"doi-asserted-by": "crossref",
"first-page": "266",
"journal-title": "J. Steroid Biochem. Mol. Biol.",
"key": "10.1016/j.jsbmb.2020.105751_bib0200",
"volume": "177",
"year": "2018"
},
{
"DOI": "10.1002/jbmr.3884",
"article-title": "Vitamin D metabolism revised: fall of dogmas",
"author": "Bouillon",
"doi-asserted-by": "crossref",
"first-page": "1985",
"journal-title": "J. Bone Miner. Res.",
"key": "10.1016/j.jsbmb.2020.105751_bib0205",
"volume": "34",
"year": "2019"
},
{
"DOI": "10.1164/rccm.201909-1867OC",
"article-title": "Vitamin D metabolism is dysregulated in Asthma and chronic obstructive pulmonary disease",
"author": "Jolliffe",
"doi-asserted-by": "crossref",
"journal-title": "Am. J. Respir. Crit. Care Med.",
"key": "10.1016/j.jsbmb.2020.105751_bib0210",
"year": "2020"
},
{
"DOI": "10.1007/s00198-018-4520-y",
"article-title": "Is calcifediol better than cholecalciferol for vitamin D supplementation?",
"author": "Quesada-Gomez",
"doi-asserted-by": "crossref",
"first-page": "1697",
"journal-title": "Osteoporos. Int.",
"key": "10.1016/j.jsbmb.2020.105751_bib0215",
"volume": "29",
"year": "2018"
},
{
"article-title": "Dexamethasone in hospitalized patients with Covid-19 - preliminary report",
"author": "RECOVERY Collaborative Group",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.jsbmb.2020.105751_bib0220",
"year": "2020"
},
{
"key": "10.1016/j.jsbmb.2020.105751_bib0225",
"unstructured": "Corticosteroids (including dexamethasone). NIH website. Updated July 17, 2020. Accessed August, 2020. https://www.covid19treatmentguidelines.nih.gov/dexamethasone/."
}
],
"reference-count": 45,
"references-count": 45,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0960076020302764"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Cell Biology",
"Clinical Biochemistry",
"Endocrinology",
"Molecular Biology",
"Molecular Medicine",
"Biochemistry",
"Endocrinology, Diabetes and Metabolism"
],
"subtitle": [],
"title": "“Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study”",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "203"
}
