Risk of Post-COVID-19 Conditions Among Adolescents and Adults Who Received Nirmatrelvir-Ritonavir for Acute COVID-19: A Retrospective Cohort Study
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf567, May 2025 (preprint)
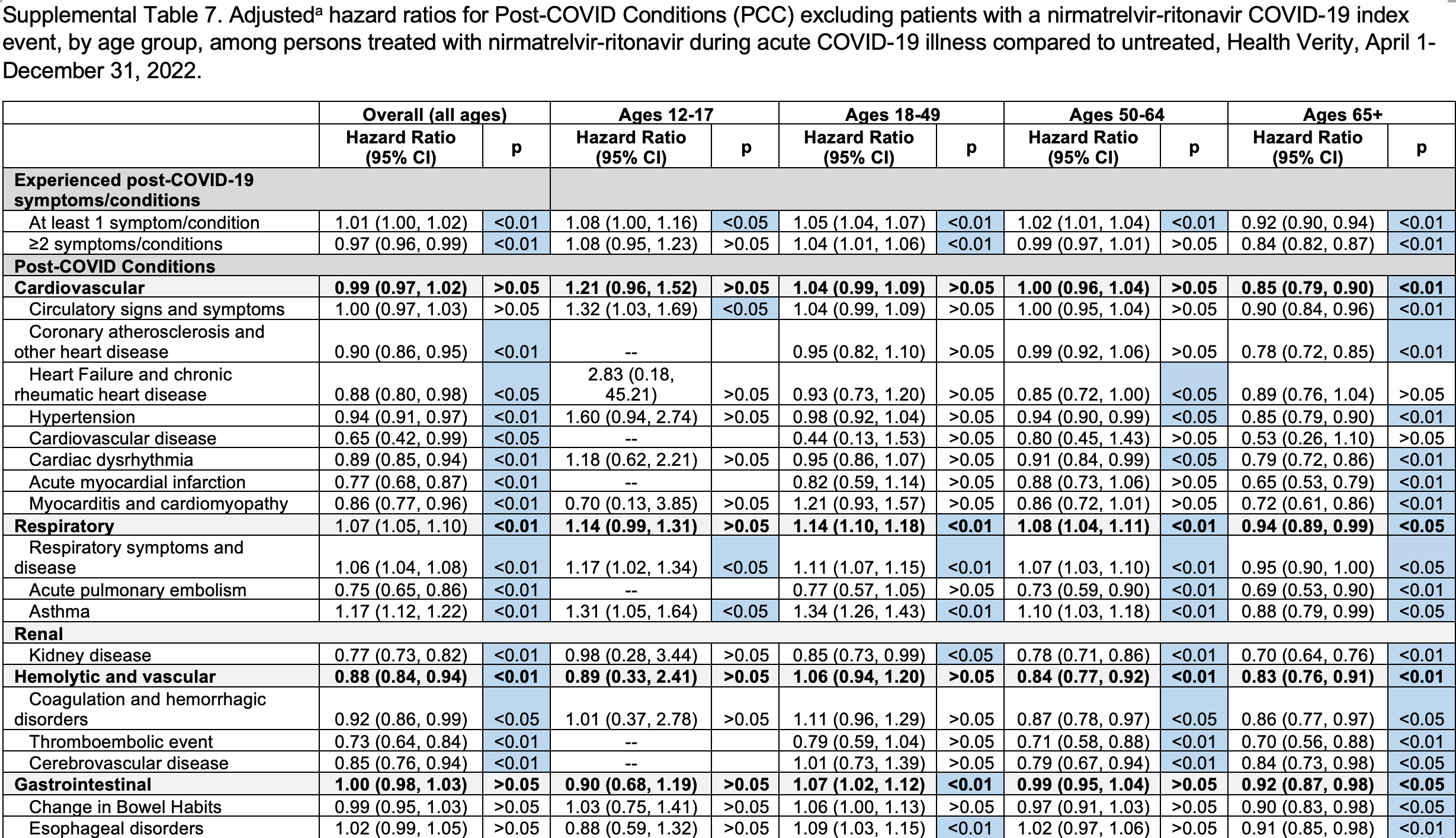
Retrospective 291,433 paxlovid recipients matched 1:2 to 582,866 untreated COVID-19 outpatients in the USA reporting a modest reduction in long COVID in the primary model for patients 50+. Analysis requiring a positive laboratory test or ICD-10 diagnosis, removing prescription-only cases solely in the treated arm and thereby eliminating asymmetric misclassification, shows overall HR 1.01 and benefit only for adults ≥65. As paxlovid’s antiviral mechanism is not age-dependent, the age-restricted benefit may reflect residual healthy-user bias whereby healthier, more proactive seniors are more inclined and able to seek treatment, as often seen in other studies. These more health-conscious patients are also more likely to take other steps to reduce risk along with other non-prescription treatments.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments18.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of long COVID, 1.0% higher, RR 1.01, p = 0.048, adjusted per study, all patients, 1+ PCC, positive test or ICD-10 diagnosis.
|
|
risk of long COVID, 8.0% higher, RR 1.08, p = 0.04, adjusted per study, 12-17, 1+ PCC, positive test or ICD-10 diagnosis.
|
|
risk of long COVID, 5.0% higher, RR 1.05, p < 0.001, adjusted per study, 18-49, 1+ PCC, positive test or ICD-10 diagnosis.
|
|
risk of long COVID, 2.0% higher, RR 1.02, p = 0.008, adjusted per study, 50-64, 1+ PCC, positive test or ICD-10 diagnosis.
|
|
risk of long COVID, 8.0% lower, RR 0.92, p < 0.001, adjusted per study, 65+, 1+ PCC, positive test or ICD-10 diagnosis.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Dalton et al., 31 May 2025, retrospective, USA, peer-reviewed, 9 authors, study period 1 April, 2022 - 31 December, 2022.
Contact: adalton@cdc.gov.
Risk of Post-COVID-19 Conditions Among Adolescents and Adults Who Received Nirmatrelvir-Ritonavir for Acute COVID-19: A Retrospective Cohort Study
Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf567
Background. Post-COVID-19 Conditions (PCC) potentially affect millions of people, but it is unclear whether treating acute COVID-19 with nirmatrelvir-ritonavir may reduce the risk of PCC. Methods. This is a retrospective cohort study using real-world, closed claims data to assess the relationship between nirmatrelvir-ritonavir and PCC by age group (12-17, 18-49, 50-64, ≥65 years). Eligible patients had a COVID-19 index date (positive laboratory test, ICD-10 diagnosis code, or nirmatrelvir-ritonavir prescription) from 1 April to 31 August 2022, in the outpatient, telehealth, or emergency department setting, and had a higher risk of severe COVID-19 based on age (≥50 years) or underlying risk factors. Treated patients (ie, received a nirmatrelvir-ritonavir prescription within ±5 days of index date) were matched 1:2 on age, sex, month of index date, and HHS region with untreated patients. PCC was defined by the presence of ≥1 of 45 new-onset symptoms or conditions recorded ≥60 days after index date. Results. Of the treated patients, 291 433 were matched to 582 866 untreated patients. Treatment with nirmatrelvir-ritonavir reduced PCC risk in adults 50-64 years (adjusted hazard ratio [aHR] 0.93, 95% confidence interval [CI] 0.92-0.95) and ≥65 years (aHR 0.88, 95% CI 0.87-0.90). Treatment had minimal effect among high-risk adults 18-49 years (aHR 0.98, 95% CI 0.97-0.99) and no effect among high-risk adolescents 12-17 years (aHR 1.06, 95% CI 0.66-1.13). Conclusions. Results using real-world data suggest a protective relationship between nirmatrelvir-ritonavir during acute illness and PCC risk among older adults, but not among adolescents. Consideration may be given to outpatient treatment of mild to moderate COVID-19 with nirmatrelvir-ritonavir to reduce the risk of severe disease and PCC.
Supplementary Data Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Patient consent statement. This analysis utilized secondary, deidentified closed claims data and was deemed not research. Patient consent was therefore not required. Financial support. The authors report no funding sources. Potential conflicts of interest. All authors: No reported conflicts.
References
Adjaye-Gbewonyo, Vahratian, Perrine, Bertolli, Long COVID in adults: United States, 2022, NCHS Data Brief
Amani, Amani, Efficacy and safety of nirmatrelvir/ritonavir (Paxlovid) for COVID-19: a rapid review and meta-analysis, J Med Virol
Arbel, Sagy, Hoshen, Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge, N Engl J Med
Bai, Du, Wang, Public health impact of Paxlovid as treatment for COVID-19, United States, Emerg Infect Dis
Berry, Kong, Paredes, Risk of long COVID in hospitalized individuals treated with remdesivir for acute COVID-19, Sci Rep
Boglione, Meli, Poletti, Risk factors and incidence of long-COVID syndrome in hospitalized patients: does remdesivir have a protective effect, Qjm
Bull-Otterson, Baca, Saydah, Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥65 years-United States, March 2020-November 2021, MMWR Morb Mortal Wkly Rep
Charlson, Pompei, Ales, Mackenzie, A new method of classifying prognostic comorbidity in longitudinal studies: development and validation, J Chronic Dis
Chuang, Wu, Liu, Efficacy of nirmatrelvir and ritonavir for postacute COVID-19 sequelae beyond 3 months of SARS-CoV-2 infection, J Med Virol
Congdon, Narrowe, Yone, Nirmatrelvir/ritonavir and risk of long COVID symptoms: a retrospective cohort study, Sci Rep
Dryden-Peterson, Kim, Kim, Nirmatrelvir plus ritonavir for early COVID-19 in a large U.S. Health system: a population-based cohort study, Ann Intern Med
Durstenfeld, Peluso, Lin, Association of nirmatrelvir for acute SARS-CoV-2 infection with subsequent long COVID symptoms in an observational cohort study, J Med Virol
Geng, Bonilla, Hedlin, Nirmatrelvir-ritonavir and symptoms in adults with postacute sequelae of SARS-CoV-2 infection: the STOP-PASC randomized clinical trial, JAMA Intern Med
Glasheen, Cordier, Gumpina, Haugh, Davis, Charlson comorbidity Index: ICD-9 update and ICD-10 translation, Am Health Drug Benefits
Hammond, Fountaine, Yunis, Nirmatrelvir for vaccinated or unvaccinated adult outpatients with Covid-19, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med
Hernandez-Romieu, Carton, Saydah, Prevalence of select new symptoms and conditions among persons aged younger than 20 years and 20 years or older at 31 to 150 days after testing positive or negative for SARS-CoV-2, JAMA Netw Open
Ioannou, Berry, Rajeevan, Effectiveness of nirmatrelvir-ritonavir against the development of post-COVID-19 conditions among U.S. Veterans: a target trial emulation, Ann Intern Med
Jones, Andrews, Dalton, Tracking the burden, distribution, and impact of post-COVID conditions in diverse populations for children, adolescents, and adults (track PCC): passive and active surveillance protocols, BMC Public Health
Joo, Kim, Huh, Risk of postacute sequelae of COVID-19 and oral antivirals in adults aged over 60 years: a nationwide retrospective cohort study, Int J Infect Dis
Kompaniyets, Bull-Otterson, Boehmer, Post-COVID-19 symptoms and conditions among children and adolescents-United States, 1 March 2020-31 January 2022, MMWR Morb Mortal Wkly Rep
Lewnard, Mclaughlin, Malden, Effectiveness of nirmatrelvirritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system, Lancet Infect Dis
Mcgarry, Sommers, Wilcock, Grabowski, Barnett, Monoclonal antibody and oral antiviral treatment of SARS-CoV-2 infection in US nursing homes, JAMA
Mcnamara, Paxlovid Prescribing Concerns for People 65+ Revealed in Medscape Survey, Medscape
Monach, Anand, Fillmore, La, Branch-Elliman, Underuse of antiviral drugs to prevent progression to severe COVID-19-veterans health administration, march-September 2022, MMWR Morb Mortal Wkly Rep
Najjar-Debbiny, Gronich, Weber, Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients, Clin Infect Dis
Preiss, Bhatia, Aragon, Effect of Paxlovid treatment during acute COVID-19 on Long COVID onset: An EHR-based target trial emulation from the N3C and RECOVER consortia, PLoS Med
Sawano, Bhattacharjee, Caraballo, Nirmatrelvir-ritonavir versus placebo-ritonavir in individuals with long COVID in the USA (PAX LC): a double-blind, randomised, placebo-controlled, phase 2, decentralised trial, Lancet Infect Dis
Shah, Joyce, Plumb, Paxlovid associated with decreased hospitalization rate among adults with COVID-19-United States, April-September 2022, MMWR Morb Mortal Wkly Rep
Sharif-Askari, Hussain Alsayed, Sharif-Askari, Sayed Hussain, Al-Muhsen et al., Nirmatrelvir plus ritonavir reduces COVID-19 hospitalization and prevents long COVID in adult outpatients, Sci Rep
Vahratian, Adjaye-Gbewonyo, Lin, Saydah, Long COVID in children: United States, 2022, NCHS Data Brief
Wang, Zhao, Paxlovid reduces the risk of long COVID in patients six months after hospital discharge, J Med Virol
Xie, Choi, Al-Aly, Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition, JAMA Intern Med
Xie, Choi, Al-Aly, Molnupiravir and risk of post-acute sequelae of covid-19: cohort study, Bmj
DOI record:
{
"DOI": "10.1093/ofid/ofaf567",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofaf567",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Post-COVID-19 Conditions (PCC) potentially affect millions of people, but it is unclear whether treating acute COVID-19 with nirmatrelvir-ritonavir may reduce the risk of PCC.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This is a retrospective cohort study using real-world, closed claims data to assess the relationship between nirmatrelvir-ritonavir and PCC by age group (12–17, 18–49, 50–64, ≥65 years). Eligible patients had a COVID-19 index date (positive laboratory test, ICD-10 diagnosis code, or nirmatrelvir-ritonavir prescription) from 1 April to 31 August 2022, in the outpatient, telehealth, or emergency department setting, and had a higher risk of severe COVID-19 based on age (≥50 years) or underlying risk factors. Treated patients (ie, received a nirmatrelvir-ritonavir prescription within ±5 days of index date) were matched 1:2 on age, sex, month of index date, and HHS region with untreated patients. PCC was defined by the presence of ≥1 of 45 new-onset symptoms or conditions recorded ≥60 days after index date.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Of the treated patients, 291 433 were matched to 582 866 untreated patients. Treatment with nirmatrelvir-ritonavir reduced PCC risk in adults 50–64 years (adjusted hazard ratio [aHR] 0.93, 95% confidence interval [CI] 0.92–0.95) and ≥65 years (aHR 0.88, 95% CI 0.87–0.90). Treatment had minimal effect among high-risk adults 18–49 years (aHR 0.98, 95% CI 0.97–0.99) and no effect among high-risk adolescents 12–17 years (aHR 1.06, 95% CI 0.66–1.13).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Results using real-world data suggest a protective relationship between nirmatrelvir-ritonavir during acute illness and PCC risk among older adults, but not among adolescents. Consideration may be given to outpatient treatment of mild to moderate COVID-19 with nirmatrelvir-ritonavir to reduce the risk of severe disease and PCC.</jats:p>\n </jats:sec>",
"article-number": "ofaf567",
"author": [
{
"ORCID": "https://orcid.org/0000-0003-3407-2961",
"affiliation": [
{
"name": "National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention , Atlanta, Georgia, USA"
}
],
"authenticated-orcid": false,
"family": "Dalton",
"given": "Alexandra F",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0009-0006-4836-1018",
"affiliation": [
{
"name": "Office of Public Health Data, Surveillance, and Technology, Centers for Disease Control and Prevention , Atlanta, Georgia, USA"
}
],
"authenticated-orcid": false,
"family": "Baca",
"given": "Sarah",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0009-0006-1840-6991",
"affiliation": [
{
"name": "Office of Public Health Data, Surveillance, and Technology, Centers for Disease Control and Prevention , Atlanta, Georgia, USA"
}
],
"authenticated-orcid": false,
"family": "Raykin",
"given": "Julia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention , Atlanta, Georgia, USA"
}
],
"family": "Gregory",
"given": "Cria O",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Office of Public Health Data, Surveillance, and Technology, Centers for Disease Control and Prevention , Atlanta, Georgia, USA"
}
],
"family": "Boehmer",
"given": "Tegan",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7184-2107",
"affiliation": [
{
"name": "National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention , Atlanta, Georgia, USA"
}
],
"authenticated-orcid": false,
"family": "Koumans",
"given": "Emilia H",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention , Atlanta, Georgia, USA"
}
],
"family": "Patel",
"given": "Priti R",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1386-8194",
"affiliation": [
{
"name": "National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention , Atlanta, Georgia, USA"
}
],
"authenticated-orcid": false,
"family": "Patel",
"given": "Pragna",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-6350-5300",
"affiliation": [
{
"name": "National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention , Atlanta, Georgia, USA"
}
],
"authenticated-orcid": false,
"family": "Saydah",
"given": "Sharon",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
9,
13
]
],
"date-time": "2025-09-13T11:45:16Z",
"timestamp": 1757763916000
},
"deposited": {
"date-parts": [
[
2025,
9,
29
]
],
"date-time": "2025-09-29T11:32:48Z",
"timestamp": 1759145568000
},
"indexed": {
"date-parts": [
[
2025,
9,
29
]
],
"date-time": "2025-09-29T12:10:09Z",
"timestamp": 1759147809609,
"version": "3.44.0"
},
"is-referenced-by-count": 0,
"issue": "10",
"issued": {
"date-parts": [
[
2025,
9,
16
]
]
},
"journal-issue": {
"issue": "10",
"published-print": {
"date-parts": [
[
2025,
9,
29
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofaf567/64284722/ofaf567.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/article-pdf/12/10/ofaf567/64284722/ofaf567.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/article-pdf/12/10/ofaf567/64284722/ofaf567.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2025,
9,
16
]
]
},
"published-online": {
"date-parts": [
[
2025,
9,
16
]
]
},
"published-other": {
"date-parts": [
[
2025,
10
]
]
},
"published-print": {
"date-parts": [
[
2025,
9,
29
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"author": "Centers for Disease Control and Prevention",
"key": "2025092907320284900_ofaf567-B1"
},
{
"article-title": "Long COVID in adults: United States, 2022",
"author": "Adjaye-Gbewonyo",
"first-page": "1",
"journal-title": "NCHS Data Brief",
"key": "2025092907320284900_ofaf567-B2",
"volume": "(480)",
"year": "2023"
},
{
"article-title": "Long COVID in children: United States, 2022",
"author": "Vahratian",
"first-page": "1",
"journal-title": "NCHS Data Brief",
"key": "2025092907320284900_ofaf567-B3",
"volume": "(479)",
"year": "2023"
},
{
"author": "Centers for Disease Control and Prevention",
"key": "2025092907320284900_ofaf567-B4"
},
{
"DOI": "10.3201/eid3002.230835",
"article-title": "Public health impact of Paxlovid as treatment for COVID-19, United States",
"author": "Bai",
"doi-asserted-by": "crossref",
"first-page": "262",
"journal-title": "Emerg Infect Dis",
"key": "2025092907320284900_ofaf567-B5",
"volume": "30",
"year": "2024"
},
{
"DOI": "10.1093/cid/ciac443",
"article-title": "Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients",
"author": "Najjar-Debbiny",
"doi-asserted-by": "crossref",
"first-page": "e342",
"journal-title": "Clin Infect Dis",
"key": "2025092907320284900_ofaf567-B6",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.15585/mmwr.mm7148e2",
"article-title": "Paxlovid associated with decreased hospitalization rate among adults with COVID-19—United States, April-September 2022",
"author": "Shah",
"doi-asserted-by": "crossref",
"first-page": "1531",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2025092907320284900_ofaf567-B7",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.7326/M22-2141",
"article-title": "Nirmatrelvir plus ritonavir for early COVID-19 in a large U.S. Health system: a population-based cohort study",
"author": "Dryden-Peterson",
"doi-asserted-by": "crossref",
"first-page": "77",
"journal-title": "Ann Intern Med",
"key": "2025092907320284900_ofaf567-B8",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(23)00118-4",
"article-title": "Effectiveness of nirmatrelvir-ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system",
"author": "Lewnard",
"doi-asserted-by": "crossref",
"first-page": "806",
"journal-title": "Lancet Infect Dis",
"key": "2025092907320284900_ofaf567-B9",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "2025092907320284900_ofaf567-B10",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2204919",
"article-title": "Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge",
"author": "Arbel",
"doi-asserted-by": "crossref",
"first-page": "790",
"journal-title": "N Engl J Med",
"key": "2025092907320284900_ofaf567-B11",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1002/jmv.28441",
"article-title": "Efficacy and safety of nirmatrelvir/ritonavir (Paxlovid) for COVID-19: a rapid review and meta-analysis",
"author": "Amani",
"doi-asserted-by": "crossref",
"first-page": "e28441",
"journal-title": "J Med Virol",
"key": "2025092907320284900_ofaf567-B12",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1038/s41598-024-76472-0",
"article-title": "Nirmatrelvir plus ritonavir reduces COVID-19 hospitalization and prevents long COVID in adult outpatients",
"author": "Saheb Sharif-Askari",
"doi-asserted-by": "crossref",
"first-page": "25901",
"journal-title": "Sci Rep",
"key": "2025092907320284900_ofaf567-B13",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.1002/jmv.29333",
"article-title": "Association of nirmatrelvir for acute SARS-CoV-2 infection with subsequent long COVID symptoms in an observational cohort study",
"author": "Durstenfeld",
"doi-asserted-by": "crossref",
"first-page": "e29333",
"journal-title": "J Med Virol",
"key": "2025092907320284900_ofaf567-B14",
"volume": "96",
"year": "2024"
},
{
"DOI": "10.1002/jmv.28750",
"article-title": "Efficacy of nirmatrelvir and ritonavir for post-acute COVID-19 sequelae beyond 3 months of SARS-CoV-2 infection",
"author": "Chuang",
"doi-asserted-by": "crossref",
"first-page": "e28750",
"journal-title": "J Med Virol",
"key": "2025092907320284900_ofaf567-B15",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.7326/M23-1394",
"article-title": "Effectiveness of nirmatrelvir-ritonavir against the development of post-COVID-19 conditions among U.S. Veterans: a target trial emulation",
"author": "Ioannou",
"doi-asserted-by": "crossref",
"first-page": "1486",
"journal-title": "Ann Intern Med",
"key": "2025092907320284900_ofaf567-B16",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1038/s41598-023-46912-4",
"article-title": "Nirmatrelvir/ritonavir and risk of long COVID symptoms: a retrospective cohort study",
"author": "Congdon",
"doi-asserted-by": "crossref",
"first-page": "19688",
"journal-title": "Sci Rep",
"key": "2025092907320284900_ofaf567-B17",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1371/journal.pmed.1004711",
"article-title": "Effect of Paxlovid treatment during acute COVID-19 on Long COVID onset: An EHR-based target trial emulation from the N3C and RECOVER consortia",
"author": "Preiss",
"doi-asserted-by": "crossref",
"first-page": "e1004711",
"issue": "9",
"journal-title": "PLoS Med",
"key": "2025092907320284900_ofaf567-B18",
"volume": "22",
"year": "2025"
},
{
"DOI": "10.1001/jamainternmed.2023.0743",
"article-title": "Association of treatment with nirmatrelvir and the risk of post–COVID-19 condition",
"author": "Xie",
"doi-asserted-by": "crossref",
"first-page": "554",
"journal-title": "JAMA Intern Med",
"key": "2025092907320284900_ofaf567-B19",
"volume": "183",
"year": "2023"
},
{
"DOI": "10.1002/jmv.29014",
"article-title": "Paxlovid reduces the risk of long COVID in patients six months after hospital discharge",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "e29014",
"journal-title": "J Med Virol",
"key": "2025092907320284900_ofaf567-B20",
"volume": "95",
"year": "2023"
},
{
"author": "Centers for Disease Control and Prevention",
"key": "2025092907320284900_ofaf567-B21"
},
{
"DOI": "10.1001/jamanetworkopen.2021.47053",
"article-title": "Prevalence of select new symptoms and conditions among persons aged younger than 20 years and 20 years or older at 31 to 150 days after testing positive or negative for SARS-CoV-2",
"author": "Hernandez-Romieu",
"doi-asserted-by": "crossref",
"first-page": "e2147053",
"journal-title": "JAMA Netw Open",
"key": "2025092907320284900_ofaf567-B22",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1186/s12889-024-19772-4",
"article-title": "Tracking the burden, distribution, and impact of post-COVID conditions in diverse populations for children, adolescents, and adults (track PCC): passive and active surveillance protocols",
"author": "Jones",
"doi-asserted-by": "crossref",
"first-page": "2345",
"journal-title": "BMC Public Health",
"key": "2025092907320284900_ofaf567-B23",
"volume": "24",
"year": "2024"
},
{
"DOI": "10.15585/mmwr.mm7121e1",
"article-title": "Post–COVID conditions among adult COVID-19 survivors aged 18–64 and ≥65 years—United States, March 2020–November 2021",
"author": "Bull-Otterson",
"doi-asserted-by": "crossref",
"first-page": "713",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2025092907320284900_ofaf567-B24",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7131a3",
"article-title": "Post-COVID-19 symptoms and conditions among children and adolescents—United States, 1 March 2020-31 January 2022",
"author": "Kompaniyets",
"doi-asserted-by": "crossref",
"first-page": "993",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2025092907320284900_ofaf567-B25",
"volume": "71",
"year": "2022"
},
{
"author": "U.S. Department of Health and Human Services",
"key": "2025092907320284900_ofaf567-B26"
},
{
"DOI": "10.1016/0021-9681(87)90171-8",
"article-title": "A new method of classifying prognostic comorbidity in longitudinal studies: development and validation",
"author": "Charlson",
"doi-asserted-by": "crossref",
"first-page": "373",
"journal-title": "J Chronic Dis",
"key": "2025092907320284900_ofaf567-B27",
"volume": "40",
"year": "1987"
},
{
"article-title": "Charlson comorbidity Index: ICD-9 update and ICD-10 translation",
"author": "Glasheen",
"first-page": "188",
"journal-title": "Am Health Drug Benefits",
"key": "2025092907320284900_ofaf567-B28",
"volume": "12",
"year": "2019"
},
{
"author": "Centers for Disease Control and Prevention",
"key": "2025092907320284900_ofaf567-B29"
},
{
"DOI": "10.1056/NEJMoa2309003",
"article-title": "Nirmatrelvir for vaccinated or unvaccinated adult outpatients with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1186",
"journal-title": "N Engl J Med",
"key": "2025092907320284900_ofaf567-B30",
"volume": "390",
"year": "2024"
},
{
"DOI": "10.1001/jamainternmed.2024.2007",
"article-title": "Nirmatrelvir-ritonavir and symptoms in adults with postacute sequelae of SARS-CoV-2 infection: the STOP-PASC randomized clinical trial",
"author": "Geng",
"doi-asserted-by": "crossref",
"first-page": "1024",
"journal-title": "JAMA Intern Med",
"key": "2025092907320284900_ofaf567-B31",
"volume": "184",
"year": "2024"
},
{
"DOI": "10.1016/S1473-3099(25)00073-8",
"article-title": "Nirmatrelvir-ritonavir versus placebo-ritonavir in individuals with long COVID in the USA (PAX LC): a double-blind, randomised, placebo-controlled, phase 2, decentralised trial",
"author": "Sawano",
"doi-asserted-by": "crossref",
"first-page": "936",
"journal-title": "Lancet Infect Dis",
"key": "2025092907320284900_ofaf567-B32",
"volume": "25",
"year": "2025"
},
{
"DOI": "10.1016/j.ijid.2025.107850",
"article-title": "Risk of postacute sequelae of COVID-19 and oral antivirals in adults aged over 60 years: a nationwide retrospective cohort study",
"author": "Joo",
"doi-asserted-by": "crossref",
"first-page": "107850",
"journal-title": "Int J Infect Dis",
"key": "2025092907320284900_ofaf567-B33",
"volume": "154",
"year": "2025"
},
{
"DOI": "10.1136/bmj-2022-074572",
"article-title": "Molnupiravir and risk of post-acute sequelae of covid-19: cohort study",
"author": "Xie",
"doi-asserted-by": "crossref",
"first-page": "e074572",
"journal-title": "Bmj",
"key": "2025092907320284900_ofaf567-B34",
"volume": "381",
"year": "2023"
},
{
"DOI": "10.1093/qjmed/hcab297",
"article-title": "Risk factors and incidence of long-COVID syndrome in hospitalized patients: does remdesivir have a protective effect?",
"author": "Boglione",
"doi-asserted-by": "crossref",
"first-page": "865",
"journal-title": "Qjm",
"key": "2025092907320284900_ofaf567-B35",
"volume": "114",
"year": "2022"
},
{
"DOI": "10.1038/s41598-025-06052-3",
"article-title": "Risk of long COVID in hospitalized individuals treated with remdesivir for acute COVID-19",
"author": "Berry",
"doi-asserted-by": "crossref",
"first-page": "27441",
"journal-title": "Sci Rep",
"key": "2025092907320284900_ofaf567-B36",
"volume": "15",
"year": "2025"
},
{
"DOI": "10.1001/jama.2023.12945",
"article-title": "Monoclonal antibody and oral antiviral treatment of SARS-CoV-2 infection in US nursing homes",
"author": "McGarry",
"doi-asserted-by": "crossref",
"first-page": "561",
"journal-title": "JAMA",
"key": "2025092907320284900_ofaf567-B37",
"volume": "330",
"year": "2023"
},
{
"author": "McNamara",
"key": "2025092907320284900_ofaf567-B38"
},
{
"DOI": "10.15585/mmwr.mm7303a2",
"article-title": "Underuse of antiviral drugs to prevent progression to severe COVID-19—veterans health administration, march-September 2022",
"author": "Monach",
"doi-asserted-by": "crossref",
"first-page": "57",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2025092907320284900_ofaf567-B39",
"volume": "73",
"year": "2024"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/article/doi/10.1093/ofid/ofaf567/8255716"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Risk of Post-COVID-19 Conditions Among Adolescents and Adults Who Received Nirmatrelvir-Ritonavir for Acute COVID-19: A Retrospective Cohort Study",
"type": "journal-article",
"volume": "12"
}