Efficacy and safety of bamlanivimab in patients with COVID-19: A systematic review and meta-analysis
et al., World Journal of Virology, doi:10.5501/wjv.v13.i1.88660, Mar 2024
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
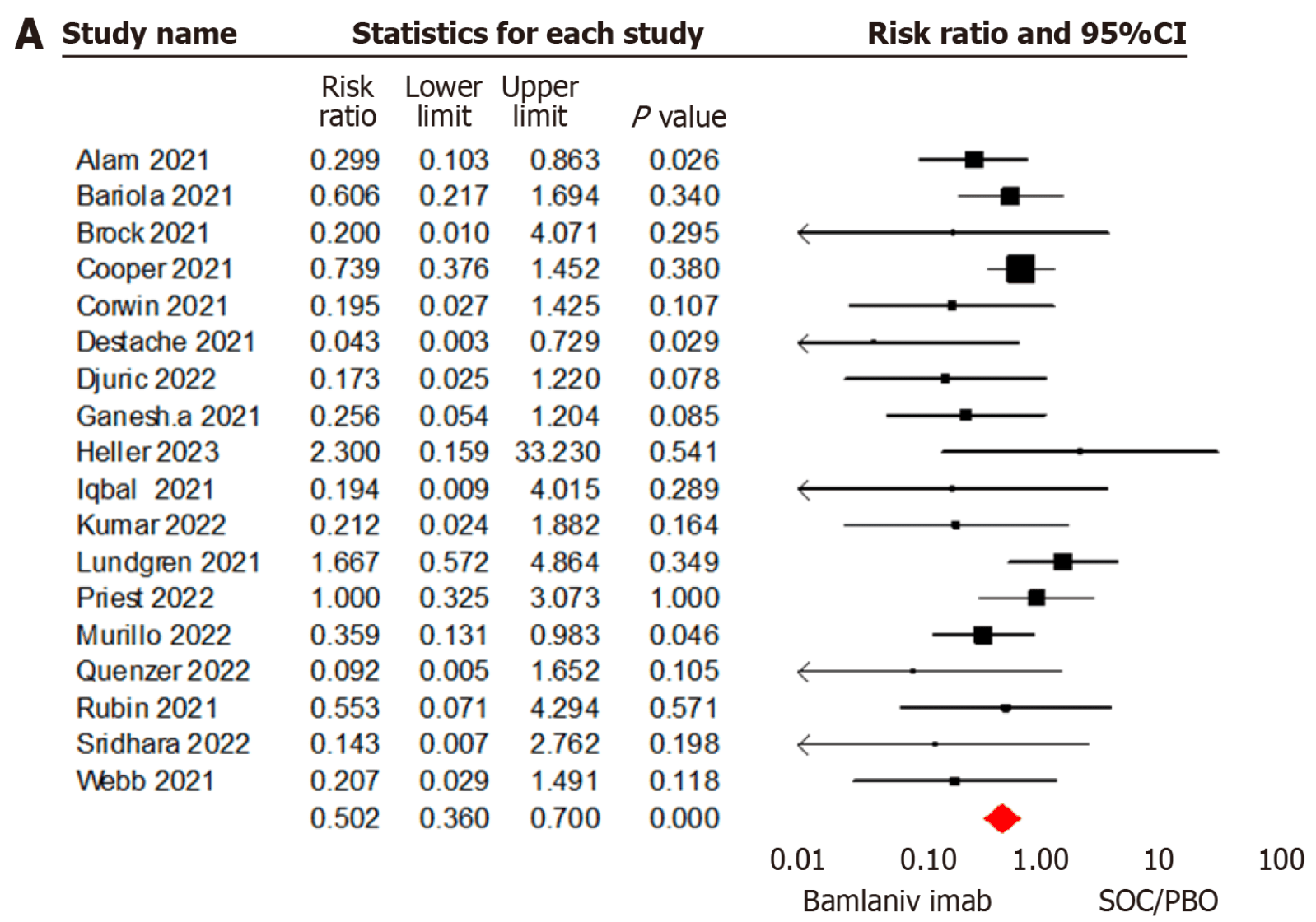
Meta-analysis of 30 studies with 47,368 COVID-19 patients showing lower mortality, hospitalization, and emergency department visits with bamlanivimab compared to standard of care/placebo.
Currently there are 22 bamlanivimab/etesevimab studies and meta-analysis shows:
| Outcome | Improvement |
|---|---|
| Mortality | 51% lower [9‑74%] |
| Ventilation | 398% higher [-76‑10142%] |
| ICU admission | 28% lower [-11‑53%] |
| Hospitalization | 42% lower [30‑53%] |
| Cases | 57% fewer [33‑72%] |
|
risk of death, 49.8% lower, RR 0.50, p < 0.001.
|
|
risk of ICU admission, 17.8% lower, RR 0.82, p = 0.30.
|
|
risk of hospitalization, 48.1% lower, RR 0.52, p < 0.001.
|
|
ER, 30.9% lower, RR 0.69, p = 0.049.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
Amani et al., 25 Mar 2024, peer-reviewed, 6 authors.
Contact: b89amani@yahoo.com.
Efficacy and safety of bamlanivimab in patients with COVID-19: A systematic review and meta-analysis
World Journal of Virology, doi:10.5501/wjv.v13.i1.88660
BACKGROUND Monoclonal antibodies (mAbs) have shown clinical benefits against coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Several studies have reported the use of bamlanivimab as a promising treatment option for COVID-19.
AIM To synthesize the latest evidence for the efficacy and safety of bamlanivimab alone in the treatment of adult patients with COVID-19.
METHODS A literature search was conducted in PubMed, Cochrane Library, Web of Science, medRxiv, and Google Scholar using "SARS-CoV-2", "COVID-19", "LY-CoV555", and "Bamlanivimab" keywords up to January 25, 2023. The quality of included studies was assessed using the Cochrane bias tools. The Comprehensive Meta-Analysis software version 3.0 was used to analyze the data.
RESULTS A total of 30 studies involving 47368 patients were included. A significant Amani B et al. Bamlanivimab for COVID-19 WJV https://www.wjgnet.com
References
Alam, Mahmud, Aggarwal, Fathma, Mahi et al., Clinical Impact of the Early Use of Monoclonal Antibody LY-CoV555 (Bamlanivimab) on Mortality and Hospitalization Among Elderly Nursing Home Patients: A Multicenter Retrospective Study, Cureus, doi:10.7759/cureus.14933
Amani, Bamlanivimab for COVID-19 WJV, doi:10.1172/JCI151697
Bariola, Mccreary, Wadas, Kip, Marroquin et al., Impact of Bamlanivimab Monoclonal Antibody Treatment on Hospitalization and Mortality Among Nonhospitalized Adults With Severe Acute Respiratory Syndrome Coronavirus 2 Infection, Open Forum Infect Dis, doi:10.1093/ofid/ofab254
Beeraka, Sukocheva, Lukina, Liu, Development of antibody resistance in emerging mutant strains of SARS CoV-2: Impediment for COVID-19 vaccines, Rev Med Virol, doi:10.1002/rmv.2346
Boaventura, Macedo, Ribeiro, Jaconiano, Soares, Post-COVID-19 Condition: Where Are We Now?, Life, doi:10.3390/life12040517
Brock, Dagher, Wechsler, Lipe, Chaftari et al., Use of Bamlanivimab in Cancer Patients with Mild-to-Moderate COVID-19, Open Forum Infect Dis, doi:10.1093/ofid/ofab466.741
Bull-Otterson, Baca, Saydah, Boehmer, Adjei et al., Post-COVID conditions among adult COVID-19 survivors aged 18-64 and≥ 65 years-United States, March 2020-November 2021, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7121e1
Center, Suite 160
Ceramella, Iacopetta, Sinicropi, Andreu, Mariconda et al., Drugs for COVID-19: An Update, Molecules, doi:10.3390/molecules27238562
Chen, Datta, Li, Chien, Price et al., First-in-Human Study of Bamlanivimab in a Randomized Trial of Hospitalized Patients With COVID-19, Clin Pharmacol Ther, doi:10.1002/cpt.2405
Chen, Nirula, Heller, Gottlieb, Boscia et al., BLAZE-1 Investigators. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2029849
Cheng, Reyes, Satram, Birch, Gibbons et al., Real-World Effectiveness of Sotrovimab for the Early Treatment of COVID-19 During SARS-CoV-2 Delta and Omicron Waves in the USA, Infect Dis Ther, doi:10.1007/s40121-022-00755-0
Chew, Moser, Daar, Wohl, Li et al., Antiviral and clinical activity of bamlanivimab in a randomized trial of non-hospitalized adults with COVID-19, Nat Commun, doi:10.1038/s41467-022-32551-2
Choudhary, Chew, Deo, Flynn, Regan et al., ACTIV-2/A5401 Study Team. Emergence of SARS-CoV-2 escape mutations during Bamlanivimab therapy in a phase II randomized clinical trial, Nat Microbiol, doi:10.1038/s41564-022-01254-1
Cooper, Christensen, Salazar, Perez, Graviss et al., Real-world Assessment of 2879 COVID-19 Patients Treated With Monoclonal Antibody Therapy: A Propensity Score-Matched Cohort Study, Open Forum Infect Dis, doi:10.1093/ofid/ofab512
Corwin, Ender, Sahu, Durgham, Mcgorry et al., The Efficacy of Bamlanivimab in Reducing Emergency Department Visits and Hospitalizations in a Real-world Setting, Open Forum Infect Dis, doi:10.1093/ofid/ofab305
Cox, Peacock, Harvey, Hughes, Wright et al., SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies, Nat Rev Microbiol, doi:10.1038/s41579-022-00809-7
Destache, Aurit, Schmidt, Erkes, Tierney et al., Bamlanivimab use in mild-to-moderate COVID-19 disease: A matched cohort design, Pharmacotherapy, doi:10.1002/phar.2613
Djuric, Bogicevic, Pešić, Davidovic, Naumovic, MO659: First Experience of Bamlanivimab for Covid-19 Positive Haemodialysis Patients: A Case-Control Study, Nephrol Dial Transplant, doi:10.1093/ndt/gfac077.019
Dougan, Nirula, Azizad, Mocherla, Gottlieb et al., BLAZE-1 Investigators. Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19, N Engl J Med, doi:10.1056/NEJMoa2102685
Farcy, Dalley, Miro, Swalley, Sherman et al., A Comparison of SARS-COV-2 Neutralizing Antibody Therapies in High-Risk Patients with Mild to Moderate COVID-19 Disease at a Single Academic Hospital, J Emerg Med, doi:10.1016/j.jemermed.2021.07.025
Filippo, Crovetto, Bucek, Nahass, Milano et al., Comparative Efficacy of Early COVID-19 Monoclonal Antibody Therapies: A Retrospective Analysis, Open Forum Infect Dis, doi:10.1093/ofid/ofac080
Ganesh, Pawlowski, Horo, Arndt, Arndt et al., Intravenous bamlanivimab use associates with reduced hospitalization in high-risk patients with mild to moderate COVID-19, J Clin Invest
Ganesh, Philpot, Bierle, Anderson, Arndt et al., Real-World Clinical Outcomes of Bamlanivimab and Casirivimab-Imdevimab Among High-Risk Patients With Mild to Moderate Coronavirus Disease 2019, J Infect Dis, doi:10.1093/infdis/jiab377
Gottlieb, Nirula, Chen, Boscia, Heller et al., Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial, N Engl J Med, doi:10.1056/NEJMoa2033130
Heller, Henrici, Büttner, Leube, Treske et al., SARS-CoV-2 neutralizing antibody therapies: an early retrospective cohort study of 26 hospitalized patients treated with bamlanivimab or casirivimab/imdevimab, Int J Infect Dis, doi:10.1016/j.ijid.2023.01.012
Higgins, Altman, Gøtzsche, Jüni, Moher et al., The Cochrane Collaboration's tool for assessing risk of bias in randomised trials, BMJ, doi:10.1136/bmj.d5928
Hoffmann, Hofmann-Winkler, Krüger, Kempf, Nehlmeier et al., SARS-CoV-2 variant B.1.617 is resistant to bamlanivimab and evades antibodies induced by infection and vaccination, Cell Rep, doi:10.1016/j.celrep.2021.109415
Huang, Mccreary, Bariola, Minnier, Wadas et al., Effectiveness of Casirivimab-Imdevimab and Sotrovimab During a SARS-CoV-2 Delta Variant Surge: A Cohort Study and Randomized Comparative Effectiveness Trial, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.20957
Iqbal, Terlau, Hernandez, Woods, Efficacy of Bamlanivimab in Reducing Hospitalization and Mortality Rates in COVID-19 Patients in a Rural Community, Cureus, doi:10.7759/cureus.16477
Karr, Chung, Burtson, Markert, Kelly, Bamlanivimab Use in a Military Treatment Facility, Mil Med, doi:10.1093/milmed/usab188
Kertes, Shapiro, David, Engel-Zohar, Rosen et al., Bamlanivimab for COVID-19 WJV
Kumar, Wu, Stosor, Moore, Achenbach et al., Real-World Experience of Bamlanivimab for Coronavirus Disease 2019 (COVID-19): A Case-Control Study, Clin Infect Dis, doi:10.1093/cid/ciab305
Lanzavecchia, Beyer, Bolo, Vaccination Is Not Enough: Understanding the Increase in Cases of COVID-19 in Chile despite a High Vaccination Rate, Epidemiologia, doi:10.3390/epidemiologia2030028
Lee, Lee, Lee, Kim, Lee et al., Regdanvimab in patients with mild-to-moderate SARS-CoV-2 infection: A propensity score-matched retrospective cohort study, Int Immunopharmacol, doi:10.1016/j.intimp.2022.108570
Lin, Hung, Lai, Wang, Chen, The impact of neutralizing monoclonal antibodies on the outcomes of COVID-19 outpatients: A systematic review and meta-analysis of randomized controlled trials, J Med Virol, doi:10.1002/jmv.27623
Liu, Arase, Neutralizing and enhancing antibodies against SARS-CoV-2, Inflamm Regen, doi:10.1186/s41232-022-00233-7
Mccreary, Bariola, Minnier, Wadas, Shovel et al., A learning health system randomized trial of monoclonal antibodies for COVID-19, doi:10.1101/2021.09.03.21262551
Moher, Liberati, Tetzlaff, Altman, Group, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement, PLoS Med, doi:10.1371/journal.pmed.1000097
Monday, Brar, Alangaden, Ramesh, SARS-CoV-2 neutralizing antibodies for COVID-19: Outcomes for bamlanivimab versus bamlanivimab-etesevimab combination in a racially diverse cohort of patients with significant comorbidities, J Clin Pharm Ther, doi:10.1111/jcpt.13694
Murillo, Lomiguen, Terrell, King, Lin et al., Effect of SARS CoV2-Neutralizing Monoclonal Antibody on Hospitalization and Mortality in Long-Term Care Facility Residents, Aging Dis, doi:10.14336/AD.2022.0205
Peiffer-Smadja, Bridier-Nahmias, Ferré, Charpentier, Garé et al., Emergence of E484K Mutation Following Bamlanivimab Monotherapy among High-Risk Patients Infected with the Alpha Variant of SARS-CoV-2, Viruses, doi:10.3390/v13081642
Priest, Blanchette, Hekman, Maddikunta, Burleson, Bamlanivimab for the Prevention of Hospitalizations and Emergency Department Visits in SARS-CoV-2-Positive Patients in a Regional Health Care System, Infect Dis Clin Pract
Quenzer, Lafree, Grey, Singh, Smyers et al., Bamlanivimab Reduces ED Returns and Hospitalizations and May Reduce COVID-19 Burden on Low-resource Border Hospitals, West J Emerg Med, doi:10.5811/westjem.2021.10.52668
Razonable, Pawlowski, Horo, Arndt, Arndt et al., Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19, EClinicalMedicine, doi:10.1016/j.eclinm.2021.101102
Rubin, Boiarsky, Canha, Giobbie-Hurder, Liu et al., Bamlanivimab Efficacy in Older and High-BMI Outpatients With COVID-19 Selected for Treatment in a Lottery-Based Allocation Process, Open Forum Infect Dis, doi:10.1093/ofid/ofab546
Savoldi, Morra, Nardo, Cattelan, Mirandola et al., Clinical efficacy of different monoclonal antibody regimens among non-hospitalised patients with mild to moderate COVID-19 at high risk for disease progression: a prospective cohort study, Eur J Clin Microbiol Infect Dis, doi:10.1007/s10096-022-04464-x
Sridhara, Gungor, Erol, Al-Obaidi, Zangeneh et al., Lack of effectiveness of Bebtelovimab monoclonal antibody among high-risk patients with SARS-Cov-2 Omicron during BA.2, BA.2.12.1 and BA.5 subvariants dominated era, PLoS One, doi:10.1371/journal.pone.0279326
Sterne, Hernán, Reeves, Savović, Berkman et al., ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions, BMJ, doi:10.1136/bmj.i4919
Sukocheva, Maksoud, Beeraka, Madhunapantula, Sinelnikov et al., Analysis of post COVID-19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome, J Adv Res, doi:10.1016/j.jare.2021.11.013
Tao, Tzou, Pond, Ioannidis, Shafer, Susceptibility of SARS-CoV-2 Omicron Variants to Therapeutic Monoclonal Antibodies: Systematic Review and Meta-analysis, Microbiol Spectr, doi:10.1128/spectrum.00926-22
Taylor, Adams, Hufford, De La Torre, Winthrop et al., Neutralizing monoclonal antibodies for treatment of COVID-19, Nat Rev Immunol, doi:10.1038/s41577-021-00542-x
Vena, Cenderello, Balletto, Mezzogori, Barbone et al., Early Administration of Bamlanivimab in Combination with Etesevimab Increases the Benefits of COVID-19 Treatment: Real-World Experience from the Liguria Region, J Clin Med, doi:10.3390/jcm10204682
Voelker, Jerath, Monoclonal antibody infusion for COVID-19 infection: results from a tertiary referral center, J Allergy Clin Immunol, doi:10.1016/j.jaci.2021.12.639
Webb, Buckel, Vento, Butler, Grisel et al., Real-world Effectiveness and Tolerability of Monoclonal Antibody Therapy for Ambulatory Patients With Early COVID-19, Open Forum Infect Dis, doi:10.1093/ofid/ofab331
Xiang, He, Li, Cheng, Zhang et al., Bamlanivimab plus etesevimab treatment have a better outcome against COVID-19: A meta-analysis, J Med Virol, doi:10.1002/jmv.27542
Zuo, Ao, Wang, Gao, Qi, Bamlanivimab improves hospitalization and mortality rates in patients with COVID-19: A systematic review and meta-analysis, J Infect, doi:10.1016/j.jinf.2021.09.003
DOI record:
{
"DOI": "10.5501/wjv.v13.i1.88660",
"ISSN": [
"2220-3249"
],
"URL": "http://dx.doi.org/10.5501/wjv.v13.i1.88660",
"abstract": "<jats:p>BACKGROUND</jats:p>\n <jats:p>Monoclonal antibodies (mAbs) have shown clinical benefits against coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2). Several studies have reported the use of bamlanivimab as a promising treatment option for COVID-19.</jats:p>\n <jats:p>AIM</jats:p>\n <jats:p>To synthesize the latest evidence for the efficacy and safety of bamlanivimab alone in the treatment of adult patients with COVID-19.</jats:p>\n <jats:p>METHODS</jats:p>\n <jats:p>A literature search was conducted in PubMed, Cochrane Library, Web of Science, medRxiv, and Google Scholar using “SARS‑CoV‑2”, “COVID-19”, “LY-CoV555”, and “Bamlanivimab” keywords up to January 25, 2023. The quality of included studies was assessed using the Cochrane bias tools. The Comprehensive Meta-Analysis software version 3.0 was used to analyze the data.</jats:p>\n <jats:p>RESULTS</jats:p>\n <jats:p>A total of 30 studies involving 47368 patients were included. A significant difference was observed between the bamlanivimab and standard of care/placebo groups in terms of mortality rate [risk ratio (RR) = 50, 95% confidence interval (CI): 0.36-0.70], hospitalization rate (RR = 0.51; 95%CI: 0.39-0.68), and emergency department (ED) visits (RR = 0.69; 95%CI: 0.47-0.99); while the two groups exhibited no significant difference in terms of intensive care unit (ICU) admission (P > 0.05). Compared to other mAbs, bamlanivimab was associated with a higher rate of hospitalization (RR = 1.44; 95%CI: 1.07-1.94). However, no significant difference was detected between the bamlanivimab and other mAbs groups in terms of mortality rate, ICU admission, and ED (P > 0.05). The incidence of any adverse events was similar between the bamlanivimab and control groups (P > 0.05).</jats:p>\n <jats:p>CONCLUSION</jats:p>\n <jats:p>Although the results suggest the efficacy and safety of bamlanivimab in COVID-19 patients, further research is required to confirm the efficacy of this drug for the current circulating SARS-CoV-2 variants.</jats:p>",
"alternative-id": [
"88660"
],
"author": [
{
"affiliation": [],
"family": "Amani",
"given": "Behnam",
"sequence": "first"
},
{
"affiliation": [],
"family": "Khodavirdilou",
"given": "Lida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rajabkhah",
"given": "Kourosh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kardan Moghaddam",
"given": "Vida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Akbarzadeh",
"given": "Arash",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amani",
"given": "Bahman",
"sequence": "additional"
}
],
"container-title": "World Journal of Virology",
"container-title-short": "World J Virol",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
3,
11
]
],
"date-time": "2024-03-11T08:54:45Z",
"timestamp": 1710147285000
},
"deposited": {
"date-parts": [
[
2024,
3,
11
]
],
"date-time": "2024-03-11T08:55:27Z",
"timestamp": 1710147327000
},
"indexed": {
"date-parts": [
[
2024,
3,
12
]
],
"date-time": "2024-03-12T00:26:41Z",
"timestamp": 1710203201522
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
3,
25
]
]
},
"journal-issue": {
"issue": "1"
},
"link": [
{
"URL": "https://www.wjgnet.com/2220-3249/full/v13/i1/88660.htm",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.wjgnet.com/2220-3249/full/v13/i1/88660.htm",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "2060",
"original-title": [],
"prefix": "10.5501",
"published": {
"date-parts": [
[
2024,
3,
25
]
]
},
"published-online": {
"date-parts": [
[
2024,
3,
25
]
]
},
"publisher": "Baishideng Publishing Group Inc.",
"reference": [
{
"DOI": "10.3390/epidemiologia2030028",
"doi-asserted-by": "publisher",
"key": "B1"
},
{
"DOI": "10.3390/molecules27238562",
"doi-asserted-by": "publisher",
"key": "B2"
},
{
"DOI": "10.1001/jamanetworkopen.2022.20957",
"doi-asserted-by": "publisher",
"key": "B3"
},
{
"DOI": "10.1016/j.eclinm.2021.101102",
"doi-asserted-by": "publisher",
"key": "B4"
},
{
"DOI": "10.1093/cid/ciac625",
"doi-asserted-by": "publisher",
"key": "B5"
},
{
"DOI": "10.1016/j.intimp.2022.108570",
"doi-asserted-by": "publisher",
"key": "B6"
},
{
"DOI": "10.3390/jcm10204682",
"doi-asserted-by": "publisher",
"key": "B7"
},
{
"DOI": "10.7759/cureus.16477",
"doi-asserted-by": "publisher",
"key": "B8"
},
{
"DOI": "10.1038/s41577-021-00542-x",
"doi-asserted-by": "publisher",
"key": "B9"
},
{
"DOI": "10.1093/milmed/usab188",
"doi-asserted-by": "publisher",
"key": "B10"
},
{
"DOI": "10.1016/j.jemermed.2021.07.025",
"doi-asserted-by": "publisher",
"key": "B11"
},
{
"DOI": "10.1056/NEJMoa2102685",
"doi-asserted-by": "publisher",
"key": "B12"
},
{
"DOI": "10.1093/ofid/ofab254",
"doi-asserted-by": "publisher",
"key": "B13"
},
{
"DOI": "10.1002/phar.2613",
"doi-asserted-by": "publisher",
"key": "B14"
},
{
"DOI": "10.1128/spectrum.00926-22",
"doi-asserted-by": "publisher",
"key": "B15"
},
{
"DOI": "10.1371/journal.pmed.1000097",
"doi-asserted-by": "publisher",
"key": "B16"
},
{
"DOI": "10.1136/bmj.i4919",
"doi-asserted-by": "publisher",
"key": "B17"
},
{
"DOI": "10.1136/bmj.d5928",
"doi-asserted-by": "publisher",
"key": "B18"
},
{
"DOI": "10.7759/cureus.14933",
"doi-asserted-by": "publisher",
"key": "B19"
},
{
"DOI": "10.1093/ofid/ofab466.741",
"doi-asserted-by": "publisher",
"key": "B20"
},
{
"DOI": "10.1002/cpt.2405",
"doi-asserted-by": "publisher",
"key": "B21"
},
{
"DOI": "10.1056/NEJMoa2029849",
"doi-asserted-by": "publisher",
"key": "B22"
},
{
"DOI": "10.1038/s41467-022-32551-2",
"doi-asserted-by": "publisher",
"key": "B23"
},
{
"DOI": "10.1093/ofid/ofab512",
"doi-asserted-by": "publisher",
"key": "B24"
},
{
"DOI": "10.1093/ofid/ofab305",
"doi-asserted-by": "publisher",
"key": "B25"
},
{
"DOI": "10.1093/ndt/gfac077.019",
"doi-asserted-by": "publisher",
"key": "B26"
},
{
"DOI": "10.1172/JCI151697",
"doi-asserted-by": "publisher",
"key": "B27"
},
{
"DOI": "10.1093/infdis/jiab377",
"doi-asserted-by": "publisher",
"key": "B28"
},
{
"DOI": "10.1001/jama.2021.0202",
"doi-asserted-by": "publisher",
"key": "B29"
},
{
"DOI": "10.1056/NEJMoa2033130",
"doi-asserted-by": "publisher",
"key": "B30"
},
{
"DOI": "10.1016/j.ijid.2023.01.012",
"doi-asserted-by": "publisher",
"key": "B31"
},
{
"DOI": "10.1093/cid/ciab305",
"doi-asserted-by": "publisher",
"key": "B32"
},
{
"DOI": "10.1101/2021.09.03.21262551",
"doi-asserted-by": "publisher",
"key": "B33"
},
{
"DOI": "10.1111/jcpt.13694",
"doi-asserted-by": "publisher",
"key": "B34"
},
{
"DOI": "10.14336/AD.2022.0205",
"doi-asserted-by": "publisher",
"key": "B35"
},
{
"DOI": "10.1097/IPC.0000000000001130",
"doi-asserted-by": "crossref",
"key": "B36",
"unstructured": "Priest DH, Blanchette LM, Hekman AL, Maddikunta R, Burleson PE. Bamlanivimab for the Prevention of Hospitalizations and Emergency Department Visits in SARS-CoV-2-Positive Patients in a Regional Health Care System. Infect Dis Clin Pract 2022; 30: 1-4"
},
{
"DOI": "10.5811/westjem.2021.10.52668",
"doi-asserted-by": "publisher",
"key": "B37"
},
{
"DOI": "10.1093/ofid/ofab546",
"doi-asserted-by": "publisher",
"key": "B38"
},
{
"DOI": "10.1093/ofid/ofac080",
"doi-asserted-by": "publisher",
"key": "B39"
},
{
"DOI": "10.1007/s10096-022-04464-x",
"doi-asserted-by": "publisher",
"key": "B40"
},
{
"DOI": "10.1371/journal.pone.0279326",
"doi-asserted-by": "publisher",
"key": "B41"
},
{
"DOI": "10.1016/j.jaci.2021.12.639",
"doi-asserted-by": "publisher",
"key": "B42"
},
{
"DOI": "10.1093/ofid/ofab331",
"doi-asserted-by": "publisher",
"key": "B43"
},
{
"DOI": "10.1186/s41232-022-00233-7",
"doi-asserted-by": "publisher",
"key": "B44"
},
{
"DOI": "10.1002/jmv.27542",
"doi-asserted-by": "publisher",
"key": "B45"
},
{
"DOI": "10.1016/j.jinf.2021.09.003",
"doi-asserted-by": "publisher",
"key": "B46"
},
{
"DOI": "10.1007/s40121-022-00755-0",
"doi-asserted-by": "publisher",
"key": "B47"
},
{
"DOI": "10.1038/s41579-022-00809-7",
"doi-asserted-by": "publisher",
"key": "B48"
},
{
"DOI": "10.1002/jmv.27623",
"doi-asserted-by": "publisher",
"key": "B49"
},
{
"DOI": "10.3390/life12040517",
"doi-asserted-by": "publisher",
"key": "B50"
},
{
"DOI": "10.15585/mmwr.mm7121e1",
"doi-asserted-by": "publisher",
"key": "B51"
},
{
"DOI": "10.1016/j.jare.2021.11.013",
"doi-asserted-by": "publisher",
"key": "B52"
},
{
"DOI": "10.1016/j.celrep.2021.109415",
"doi-asserted-by": "publisher",
"key": "B53"
},
{
"DOI": "10.3390/v13081642",
"doi-asserted-by": "publisher",
"key": "B54"
},
{
"DOI": "10.1038/s41564-022-01254-1",
"doi-asserted-by": "publisher",
"key": "B55"
},
{
"DOI": "10.1002/rmv.2346",
"doi-asserted-by": "publisher",
"key": "B56"
}
],
"reference-count": 56,
"references-count": 56,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.wjgnet.com/2220-3249/full/v13/i1/88660.htm"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy and safety of bamlanivimab in patients with COVID-19: A systematic review and meta-analysis",
"type": "journal-article",
"volume": "13"
}
