Bamlanivimab efficacy in older and high BMI outpatients with Covid-19 selected for treatment in a lottery-based allocation process
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofab546, Nov 2021
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective database analysis of 1257 PCR+ outpatients with age ≥65, BMI≥35, 191 receiving bamlanivimab via lottery. Authors note that the alpha variant was most common during the study period, and that efficacy against other variants can be much lower. Authors note confounding due to prioritization in the lottery and differential reporting in the database.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments6.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in the after exclusion results of meta-analysis:
significant unadjusted confounding possible.
|
risk of death, 44.2% lower, RR 0.56, p = 1.00, treatment 1 of 191 (0.5%), control 10 of 1,066 (0.9%), NNT 241.
|
|
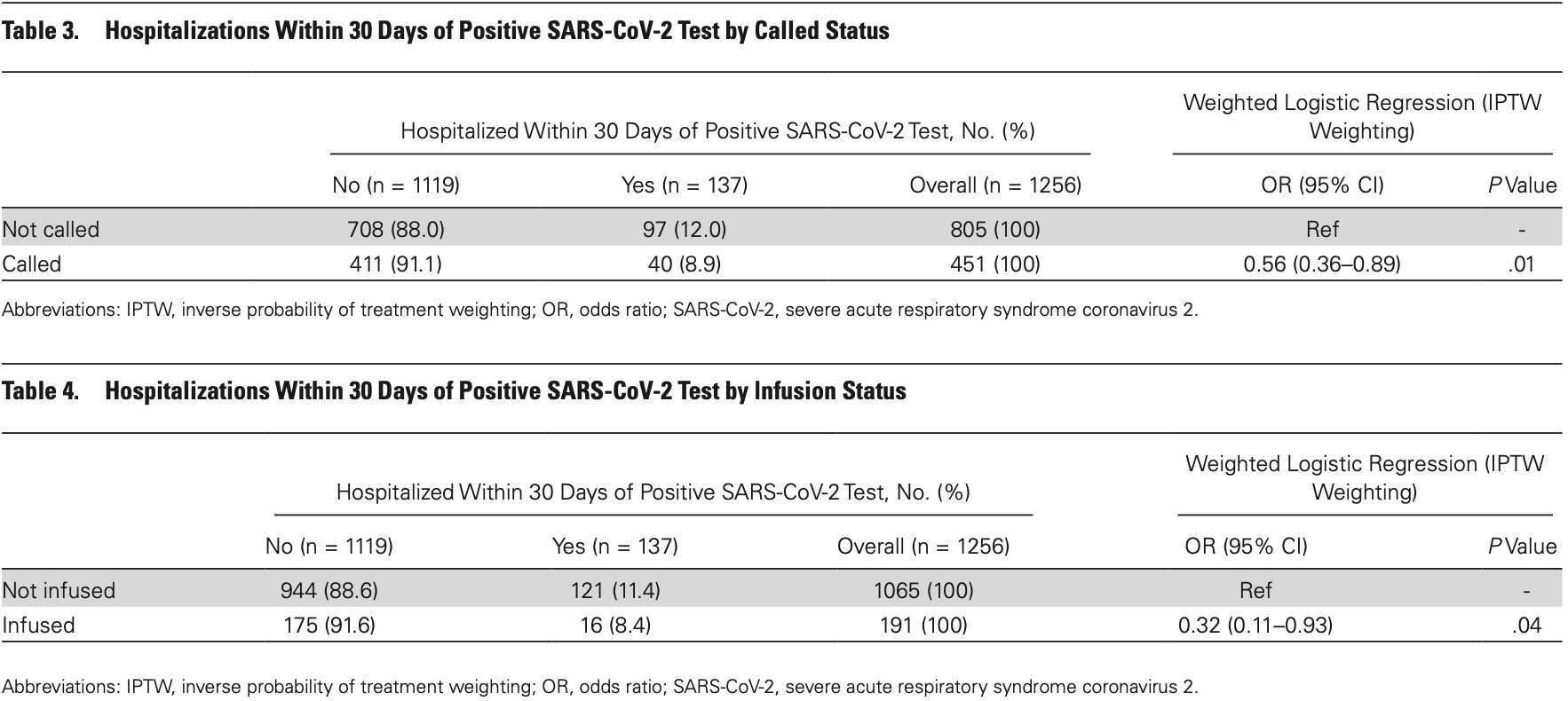
risk of hospitalization, 65.3% lower, RR 0.35, p = 0.04, treatment 16 of 191 (8.4%), control 121 of 1,065 (11.4%), odds ratio converted to relative risk, IPTW weighted logistic regression.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Conflicts of interest:
research funding from the drug patent holder, consulting for the pharmaceutical industry.
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Rubin et al., 3 Nov 2021, retrospective, USA, peer-reviewed, 7 authors, study period 9 December, 2020 - 25 February, 2021, average treatment delay 6.0 days.
Bamlanivimab Efficacy in Older and High-BMI Outpatients With COVID-19 Selected for Treatment in a Lottery-Based Allocation Process
Open Forum Infectious Diseases, doi:10.1093/ofid/ofab546
Background. Given the challenges associated with timely delivery of monoclonal antibody (mAb) therapy to outpatients with coronavirus disease 2019 (COVID-19) who are most likely to benefit, it is critical to understand the effectiveness of such therapy outside the context of clinical trials. Methods. This was a case-control study of 1257 adult outpatients with COVID-19, ≥65 years of age or with body mass index (BMI) ≥35, who were entered into a lottery for mAb therapy. Results. Patients who were called to be offered mAb therapy had a statistically significant 44% reduction in the odds of hospitalization within 30 days of a positive severe acute respiratory syndrome coronavirus 2 test compared with those who were not called (odds ratio [OR], 0.56; 95% CI, 0.36-0.89; P = .01). Patients who actually received bamlanivimab had a statistically significant 68% reduction in the odds of hospitalization compared with those who did not receive bamlanivimab (OR, 0.32; 95% CI, 0.11-0.93; P = .04). Conclusions. This study supports the effectiveness of bamlanivimab in reducing COVID-19-related hospitalizations in patients ≥65 or with BMI ≥35.
Author contributions. Dr. Rubin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
Chen, Nirula, Heller, BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med
Chen, Winkler, Case, In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains, Nature
Corwin, Ender, Sahu, The efficacy of bamlanivimab in reducing emergency department visits and hospitalizations in a real-world setting, Open Forum Infect Dis
Dougan, Nirula, Azizad, BLAZE-1 Investigators. Bamlanivimab plus etesevimab in mild or moderate Covid-19, N Engl J Med
Ganesh, Philpot, Bierle, Real-world clinical outcomes of bamlanivimab and casirivimab-imdevimab among high-risk patients with mild to moderate coronavirus disease 2019, J Infect Dis
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Jenks, Aslam, Horton, Early monoclonal antibody administration can reduce both hospitalizations and mortality in high-risk outpatients with COVID-19, Clin Infect Dis
Kumar, Wu, Sosor, Real-world experience of bamlanivimab for COVID-19: a case-control study, Clin Infect Dis
O'brien, Forleo-Neto, Musser, Covid-19 Phase 3 Prevention Trial Team. Subcutaneous REGEN-COV antibody combination to prevent Covid-19, N Engl J Med
Planas, Veyer, Baidaliuk, Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization, Nature
Rubin, Dryden-Peterson, Hammond, A novel approach to equitable distribution of scarce therapeutics: institutional experience implementing a reserve system for allocation of COVID-19 monoclonal antibodies, Chest, doi:10.1016/j.chest.2021.08.003
Verderese, Stepanova, Lam, Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19. A real-world experience, Clin Infect Dis, doi:10.1093/cid/ciab579
Webb, Buckel, Vento, Real-world effectiveness and tolerability of monoclonal antibody therapy for ambulatory patients with early COVID-19, Open Forum Infect Dis
Weinreich, Sivapalasingam, Norton, Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med
DOI record:
{
"DOI": "10.1093/ofid/ofab546",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofab546",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Given the challenges associated with timely delivery of monoclonal antibody (mAb) therapy to outpatients with coronavirus disease 2019 (COVID-19) who are most likely to benefit, it is critical to understand the effectiveness of such therapy outside the context of clinical trials.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This was a case–control study of 1257 adult outpatients with COVID-19, ≥65 years of age or with body mass index (BMI) ≥35, who were entered into a lottery for mAb therapy.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Patients who were called to be offered mAb therapy had a statistically significant 44% reduction in the odds of hospitalization within 30 days of a positive severe acute respiratory syndrome coronavirus 2 test compared with those who were not called (odds ratio [OR], 0.56; 95% CI, 0.36–0.89; P=.01). Patients who actually received bamlanivimab had a statistically significant 68% reduction in the odds of hospitalization compared with those who did not receive bamlanivimab (OR, 0.32; 95% CI, 0.11–0.93; P=.04).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>This study supports the effectiveness of bamlanivimab in reducing COVID-19-related hospitalizations in patients ≥65 or with BMI ≥35.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-1788-3618",
"affiliation": [
{
"name": "Division of Pulmonary and Critical Care Medicine, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts, USA"
}
],
"authenticated-orcid": false,
"family": "Rubin",
"given": "Emily B",
"sequence": "first"
},
{
"affiliation": [
{
"name": "University of California, Los Angeles Medical Center, Los Angeles, California, USA"
}
],
"family": "Boiarsky",
"given": "Jonathan A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Gastroenterology, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts, USA"
}
],
"family": "Canha",
"given": "Lauren A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Biostatistics, Department of Data Science, Dana-Farber Cancer Institute, Boston, Massachusetts, USA"
}
],
"family": "Giobbie-Hurder",
"given": "Anita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Biostatistics, Department of Data Science, Dana-Farber Cancer Institute, Boston, Massachusetts, USA"
}
],
"family": "Liu",
"given": "Mofei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke University Medical Center, Durham, North Carolina, USA"
}
],
"family": "Townsend",
"given": "Matthew J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Gastroenterology, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts, USA"
}
],
"family": "Dougan",
"given": "Michael",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
11,
4
]
],
"date-time": "2021-11-04T02:49:49Z",
"timestamp": 1635994189000
},
"deposited": {
"date-parts": [
[
2021,
12,
7
]
],
"date-time": "2021-12-07T19:08:23Z",
"timestamp": 1638904103000
},
"indexed": {
"date-parts": [
[
2023,
5,
8
]
],
"date-time": "2023-05-08T07:53:04Z",
"timestamp": 1683532384149
},
"is-referenced-by-count": 4,
"issue": "12",
"issued": {
"date-parts": [
[
2021,
11,
3
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2021,
12,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
11,
3
]
],
"date-time": "2021-11-03T00:00:00Z",
"timestamp": 1635897600000
}
}
],
"link": [
{
"URL": "http://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofab546/41073072/ofab546.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/article-pdf/8/12/ofab546/41640764/ofab546.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/article-pdf/8/12/ofab546/41640764/ofab546.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2021,
11,
3
]
]
},
"published-online": {
"date-parts": [
[
2021,
11,
3
]
]
},
"published-other": {
"date-parts": [
[
2021,
12,
1
]
]
},
"published-print": {
"date-parts": [
[
2021,
12,
1
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"author": "US Food and Drug Administration.",
"key": "2021120719080297900_CIT0001"
},
{
"author": "Regeneron.",
"key": "2021120719080297900_CIT0002"
},
{
"DOI": "10.1056/NEJMoa2029849",
"article-title": "SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19.",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "229",
"journal-title": "N Engl J Med",
"key": "2021120719080297900_CIT0003",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19.",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "2021120719080297900_CIT0004",
"volume": "384",
"year": "2021"
},
{
"author": "US Food and Drug Administration (FDA).",
"key": "2021120719080297900_CIT0005",
"volume-title": "Coronavirus (COVID-19) update: FDA authorizes monoclonal antibodies for treatment of COVID-19"
},
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "Bamlanivimab plus etesevimab in mild or moderate Covid-19.",
"author": "Dougan",
"doi-asserted-by": "crossref",
"first-page": "1382",
"journal-title": "N Engl J Med",
"key": "2021120719080297900_CIT0006",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2109682",
"article-title": "Subcutaneous REGEN-COV antibody combination to prevent Covid-19.",
"author": "O’Brien",
"doi-asserted-by": "crossref",
"first-page": "1184",
"journal-title": "N Engl J Med",
"key": "2021120719080297900_CIT0007",
"volume": "385",
"year": "2021"
},
{
"author": "Massachusetts Department of Public Health.",
"key": "2021120719080297900_CIT0008",
"volume-title": "Guidance for allocation of COVID-19 monoclonal antibody therapeutics"
},
{
"article-title": "A novel approach to equitable distribution of scarce therapeutics: institutional experience implementing a reserve system for allocation of COVID-19 monoclonal antibodies.",
"author": "Rubin",
"first-page": "S0012-3692(21)03622-9",
"journal-title": "Chest",
"key": "2021120719080297900_CIT0009",
"year": "2021"
},
{
"author": "United States Centers for Disease Control and Prevention.",
"key": "2021120719080297900_CIT0010",
"year": "2021"
},
{
"article-title": "Real-world experience of bamlanivimab for COVID-19: a case-control study.",
"author": "Kumar",
"journal-title": "Clin Infect Dis",
"key": "2021120719080297900_CIT0011",
"year": ""
},
{
"article-title": "Early monoclonal antibody administration can reduce both hospitalizations and mortality in high-risk outpatients with COVID-19.",
"author": "Jenks",
"journal-title": "Clin Infect Dis",
"key": "2021120719080297900_CIT0012",
"year": ""
},
{
"DOI": "10.1093/ofid/ofab331",
"article-title": "Real-world effectiveness and tolerability of monoclonal antibody therapy for ambulatory patients with early COVID-19.",
"author": "Webb",
"doi-asserted-by": "crossref",
"first-page": "XXX",
"journal-title": "Open Forum Infect Dis",
"key": "2021120719080297900_CIT0013",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1093/infdis/jiab377",
"article-title": "Real-world clinical outcomes of bamlanivimab and casirivimab-imdevimab among high-risk patients with mild to moderate coronavirus disease 2019.",
"author": "Ganesh",
"doi-asserted-by": "crossref",
"first-page": "1278",
"journal-title": "J Infect Dis",
"key": "2021120719080297900_CIT0014",
"volume": "224",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofab305",
"article-title": "The efficacy of bamlanivimab in reducing emergency department visits and hospitalizations in a real-world setting.",
"author": "Corwin",
"doi-asserted-by": "crossref",
"journal-title": "Open Forum Infect Dis",
"key": "2021120719080297900_CIT0015",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciab579",
"article-title": "Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19. A real-world experience.",
"author": "Verderese",
"doi-asserted-by": "crossref",
"first-page": "ciab579",
"journal-title": "Clin Infect Dis",
"key": "2021120719080297900_CIT0016",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial.",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "632",
"journal-title": "JAMA",
"key": "2021120719080297900_CIT0017",
"volume": "325",
"year": "2021"
},
{
"author": "US Food and Drug Administration.",
"key": "2021120719080297900_CIT0018"
},
{
"DOI": "10.1038/s41586-021-03777-9",
"article-title": "Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization.",
"author": "Planas",
"doi-asserted-by": "crossref",
"first-page": "276",
"journal-title": "Nature",
"key": "2021120719080297900_CIT0019",
"volume": "596",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03720-y",
"article-title": "In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains.",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "103",
"journal-title": "Nature",
"key": "2021120719080297900_CIT0020",
"volume": "596",
"year": "2021"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/article/doi/10.1093/ofid/ofab546/6420437"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Oncology"
],
"subtitle": [],
"title": "Bamlanivimab Efficacy in Older and High-BMI Outpatients With COVID-19 Selected for Treatment in a Lottery-Based Allocation Process",
"type": "journal-article",
"volume": "8"
}
