The Efficacy of Bamlanivimab in Reducing Emergency Department Visits and Hospitalizations in a Real-world Setting
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofab305, Jun 2021
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
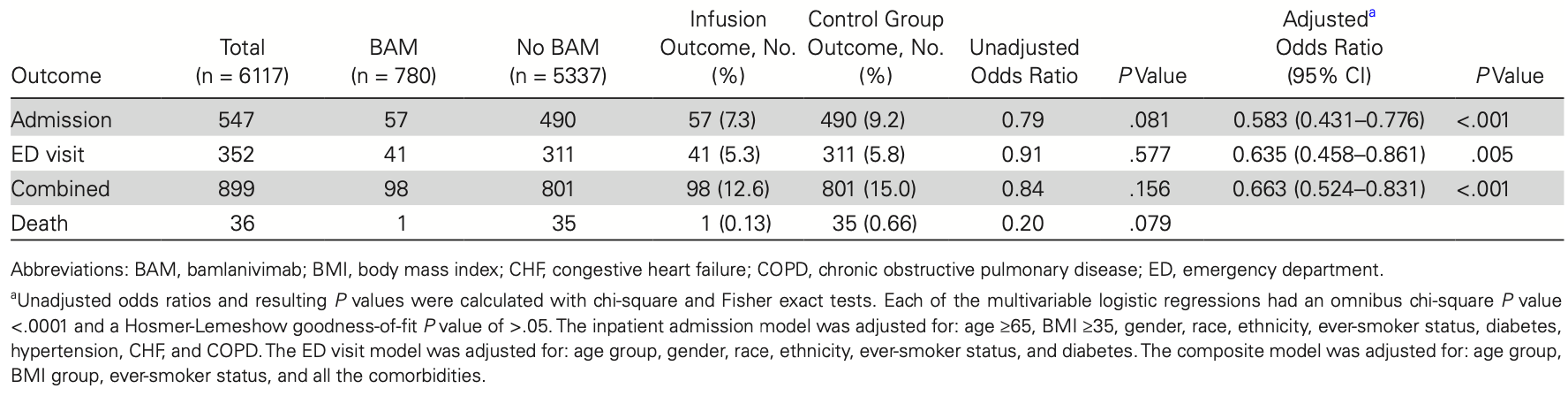
Retrospective 780 bamlanivimab patients and 5,337 patients not receiving treatment, showing lower hospitalization and ER visits with treatment.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending bamlanivimab/etesevimab also recommended them, or
because the patient seeking out bamlanivimab/etesevimab is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Therefore, these kind of studies may
overestimate efficacy.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments9.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 80.5% lower, RR 0.20, p = 0.08, treatment 1 of 780 (0.1%), control 35 of 5,337 (0.7%), NNT 190.
|
|
risk of hospitalization, 39.4% lower, RR 0.61, p < 0.001, treatment 57 of 780 (7.3%), control 490 of 5,337 (9.2%), odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
5.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
6.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
7.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Corwin et al., 10 Jun 2021, retrospective, USA, peer-reviewed, 8 authors, study period 23 November, 2020 - 17 January, 2021.
Contact: douglas.corwin@sluhn.org.
The Efficacy of Bamlanivimab in Reducing Emergency Department Visits and Hospitalizations in a Real-world Setting
Open Forum Infectious Diseases, doi:10.1093/ofid/ofab305
Bamlanivimab, a monoclonal antibody targeting the spike protein of severe acute respiratory syndrome coronavirus 2, is available for ambulatory treatment of coronavirus disease 2019 . This real-world study confirms the efficacy of bamlanivimab in reducing hospital admissions and emergency department visits among high-risk outpatients with mild to moderate COVID-19 illness and reveals a trend toward improved mortality.
Author contributions. D.S.C., P.E., N.S., R.D., and J.S. drafted the initial manuscript. All authors reviewed, contributed to the revision and approved the final manuscript. D.S.C. had full access to manuscript and final responsibility to submit for publication.
References
Bowden, Hospitals face severe shortages as pandemic grinds forward. The Hill
Chen, Nirula, Heller, BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med
Goldhill, Hospitals in half the states are facing a massive staffing shortage, STAT
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Halpin, Criner, Papi, Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD Science Committee report on COVID-19 and chronic obstructive pulmonary disease, Am J Respir Crit Care Med
Jorden, Rudman, Evidence for limited early spread of COVID-19 within the United States, MMWR Morb Mortal Wkly Rep
Kane, Shamliyan, Mueller, Nurse staffing and quality of patient care, Evid Rep Technol Assess (Full Rep)
Leatherby, Keefe, Tompkins, There's no place for them to go': I.C.U. beds near capacity across U.S. The New York Times
O'hearn, Liu, Cudhea, Coronavirus disease 2019 hospitalizations attributable to cardiometabolic conditions in the United States: a comparative risk assessment analysis, J Am Heart Assoc
Wang, Nair, Liu, Increased resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7 to antibody neutralization
Wickham, R packages for data science
Widera, Wilhelm, Hoehl, Bamlanivimab does not neutralize two SARS-CoV-2 variants carrying E484K in vitro
DOI record:
{
"DOI": "10.1093/ofid/ofab305",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofab305",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>Bamlanivimab, a monoclonal antibody targeting the spike protein of severe acute respiratory syndrome coronavirus 2, is available for ambulatory treatment of coronavirus disease 2019 (COVID-19). This real-world study confirms the efficacy of bamlanivimab in reducing hospital admissions and emergency department visits among high-risk outpatients with mild to moderate COVID-19 illness and reveals a trend toward improved mortality.</jats:p>",
"author": [
{
"affiliation": [
{
"name": "Section of Pulmonary and Critical Care, Department of Medicine, St Luke’s University Health Network, Bethlehem, Pennsylvania, USA"
}
],
"family": "Corwin",
"given": "Douglas S",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Section of Infectious Diseases, Department of Medicine, St. Luke’s University Health Network, Bethlehem, Pennsylvania, USA"
}
],
"family": "Ender",
"given": "Peter T",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Section of Pulmonary and Critical Care, Department of Medicine, St Luke’s University Health Network, Bethlehem, Pennsylvania, USA"
}
],
"family": "Sahu",
"given": "Nitasa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Temple/St. Luke’s School of Medicine, Bethlehem, Pennsylvania, USA"
}
],
"family": "Durgham",
"given": "Ryan A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Family Medicine, St. Luke’s University Health Network, Bethlehem, Pennsylvania, USA"
}
],
"family": "McGorry",
"given": "Dennis M",
"sequence": "additional",
"suffix": "Jr."
},
{
"affiliation": [
{
"name": "Section of Pulmonary and Critical Care, Department of Medicine, St Luke’s University Health Network, Bethlehem, Pennsylvania, USA"
}
],
"family": "Rahman",
"given": "Awan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Graduate Medical Education, Data Management and Outcomes Assessment, St. Luke’s University Health Network, Bethlehem, Pennsylvania, USA"
}
],
"family": "Stoltzfus",
"given": "Jill",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Section of Infectious Diseases, Department of Medicine, St. Luke’s University Health Network, Bethlehem, Pennsylvania, USA"
}
],
"family": "Jahre",
"given": "Jeffrey A",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
6,
10
]
],
"date-time": "2021-06-10T16:08:25Z",
"timestamp": 1623341305000
},
"deposited": {
"date-parts": [
[
2021,
7,
11
]
],
"date-time": "2021-07-11T19:21:36Z",
"timestamp": 1626031296000
},
"indexed": {
"date-parts": [
[
2023,
5,
8
]
],
"date-time": "2023-05-08T07:50:10Z",
"timestamp": 1683532210332
},
"is-referenced-by-count": 5,
"issue": "7",
"issued": {
"date-parts": [
[
2021,
6,
10
]
]
},
"journal-issue": {
"issue": "7",
"published-print": {
"date-parts": [
[
2021,
7,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
6,
10
]
],
"date-time": "2021-06-10T00:00:00Z",
"timestamp": 1623283200000
}
}
],
"link": [
{
"URL": "http://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofab305/38576790/ofab305.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "http://academic.oup.com/ofid/article-pdf/8/7/ofab305/38897252/ofab305.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "http://academic.oup.com/ofid/article-pdf/8/7/ofab305/38897252/ofab305.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2021,
6,
10
]
]
},
"published-online": {
"date-parts": [
[
2021,
6,
10
]
]
},
"published-other": {
"date-parts": [
[
2021,
7,
1
]
]
},
"published-print": {
"date-parts": [
[
2021,
7,
1
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.15585/mmwr.mm6922e1",
"article-title": "Evidence for limited early spread of COVID-19 within the United States, January – February 2020",
"author": "Jorden",
"doi-asserted-by": "crossref",
"first-page": "680",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2021071119212397000_CIT0001",
"volume": "69",
"year": "2020"
},
{
"key": "2021071119212397000_CIT0002"
},
{
"author": "Leatherby",
"key": "2021071119212397000_CIT0003",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2029849",
"article-title": "SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "229",
"journal-title": "N Engl J Med",
"key": "2021071119212397000_CIT0004",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "632",
"journal-title": "JAMA",
"key": "2021071119212397000_CIT0005",
"volume": "325",
"year": "2021"
},
{
"author": "Wickham",
"key": "2021071119212397000_CIT0006"
},
{
"DOI": "10.1161/JAHA.120.019259",
"article-title": "Coronavirus disease 2019 hospitalizations attributable to cardiometabolic conditions in the United States: a comparative risk assessment analysis",
"author": "O’Hearn",
"doi-asserted-by": "crossref",
"first-page": "e019259",
"journal-title": "J Am Heart Assoc",
"key": "2021071119212397000_CIT0007",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1164/rccm.202009-3533SO",
"article-title": "Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD Science Committee report on COVID-19 and chronic obstructive pulmonary disease",
"author": "Halpin",
"doi-asserted-by": "crossref",
"first-page": "24",
"journal-title": "Am J Respir Crit Care Med",
"key": "2021071119212397000_CIT0008",
"volume": "203",
"year": "2021"
},
{
"article-title": "Nurse staffing and quality of patient care",
"author": "Kane",
"first-page": "1",
"journal-title": "Evid Rep Technol Assess (Full Rep)",
"key": "2021071119212397000_CIT0009",
"year": "2007;"
},
{
"author": "Goldhill",
"key": "2021071119212397000_CIT0010"
},
{
"author": "Bowden",
"key": "2021071119212397000_CIT0011",
"year": "2021"
},
{
"key": "2021071119212397000_CIT0012",
"year": "2021"
},
{
"article-title": "Increased resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7 to antibody neutralization",
"author": "Wang",
"journal-title": "bioRxiv",
"key": "2021071119212397000_CIT0013",
"year": "2021"
},
{
"article-title": "Bamlanivimab does not neutralize two SARS-CoV-2 variants carrying E484K in vitro",
"author": "Widera",
"journal-title": "medRxiv",
"key": "2021071119212397000_CIT0014",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "632",
"journal-title": "JAMA",
"key": "2021071119212397000_CIT0015",
"volume": "325",
"year": "2021"
}
],
"reference-count": 15,
"references-count": 15,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/article/doi/10.1093/ofid/ofab305/6295837"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Oncology"
],
"subtitle": [],
"title": "The Efficacy of Bamlanivimab in Reducing Emergency Department Visits and Hospitalizations in a Real-world Setting",
"type": "journal-article",
"volume": "8"
}
