Clinical Impact of the Early Use of Monoclonal Antibody LY-CoV555 (Bamlanivimab) on Mortality and Hospitalization Among Elderly Nursing Home Patients: A Multicenter Retrospective Study
et al., Cureus, doi:10.7759/cureus.14933, May 2021
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
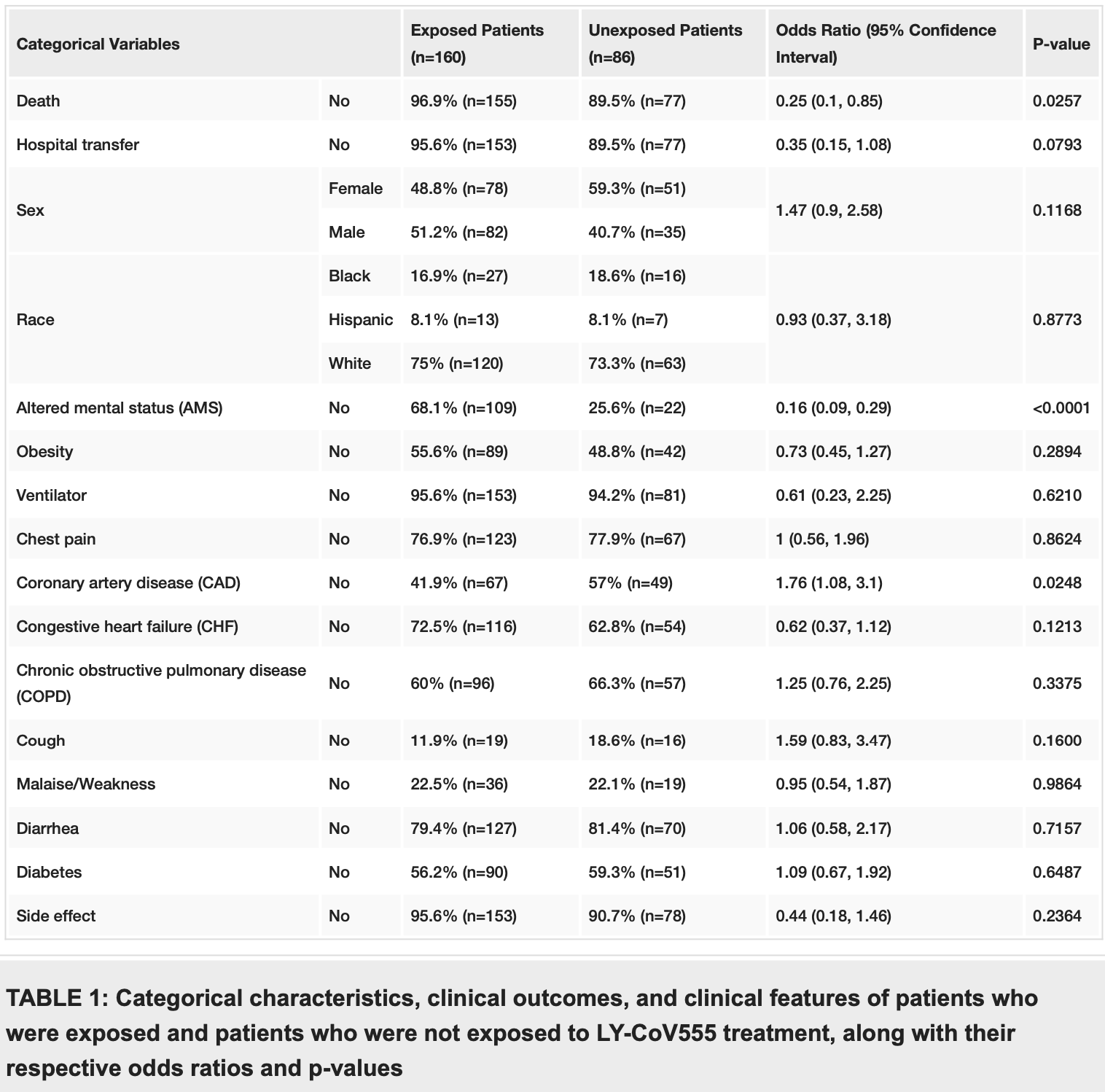
Retrospective 246 nursing home patients showing lower mortality with early bamlanivimab treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments6.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 75.0% lower, OR 0.25, p = 0.03, treatment 160, control 86, RR approximated with OR.
|
|
risk of hospitalization, 65.0% lower, OR 0.35, p = 0.08, treatment 160, control 86, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Alam et al., 10 May 2021, retrospective, USA, peer-reviewed, mean age 82.4, 9 authors, study period 15 November, 2020 - 31 January, 2021.
Contact: dr.tuku@yahoo.com.
Clinical Impact of the Early Use of Monoclonal Antibody LY-CoV555 (Bamlanivimab) on Mortality and Hospitalization Among Elderly Nursing Home Patients: A Multicenter Retrospective Study
Cureus, doi:10.7759/cureus.14933
Importance Coronavirus disease 2019 (COVID-19) outbreaks are frequent occurrences in nursing homes and long-term care facilities (LTCFs), resulting in subsequent hospitalization and death.
Rationale Virus-neutralizing monoclonal antibodies demonstrate a significant decrease in both viral load and hospital transfer rate among patients with mild-to-moderate COVID-19 infection.
Objective To assess the clinical outcomes of COVID-19 patients with mild-to-moderate symptoms in LTCFs who received LY-CoV555 as compared to those who did not receive this treatment.
Design Retrospective case-control study and logistic regression analysis.
Setting LTCFs in New York.
Participants Two-hundred forty-six (246) LTCF patients diagnosed with mild-to-moderate COVID-19 infection with positive COVID-19 polymerase chain reaction (PCR) from November 15, 2020, to January 31, 2021.
Methods Two-hundred forty-six (246) COVID-19 patients were identified from electronic medical records, out of which 160 cases were exposed to LY-CoV555 treatment (700 mg single dose, intravenous infusion). Eightysix (86) patients were unexposed controls who did not receive monoclonal antibodies, LY-CoV555.
Outcome We assessed the odds of death and hospitalization of exposed cases as compared to unexposed controls. Using logistic regression analysis, we also assessed the risk factors associated with these outcomes in the entire sample population.
Additional Information Disclosures Human subjects: Consent was obtained or waived by all participants in this study. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
References
Ahmad, Alam, Saadi, Doxycycline and hydroxychloroquine as treatment for high-risk COVID-19 patients: experience from case series of 54 patients in long-term care facilities, MedRxiv, doi:10.1101/2020.05.18.20066902?utm_medium=email&utm_source=transaction
Ahmed, Karim, Ross, A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, Int J Infect Dis, doi:10.1016/j.ijid.2020.11.191?utm_medium=email&utm_source=transaction
Alam, Mahmud, Rahman, Simpson, Aggarwal et al., Clinical outcomes of early treatment with doxycycline for 89 high-risk COVID-19 patients in long-term care facilities in New York, Cureus, doi:10.7759/cureus.9658?utm_medium=email&utm_source=transaction
Anderson, Rouphael, Widge, Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults, N Engl J Med, doi:10.1056/NEJMoa2028436?utm_medium=email&utm_source=transaction
Borba, Val, Sampaio, Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.8857?utm_medium=email&utm_source=transaction
Chen, Nirula, Heller, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2029849?utm_medium=email&utm_source=transaction
Conti, Ronconi, Caraffa, Gallenga, Ross et al., Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COV-19 or SARS-CoV-2): antiinflammatory strategies, J Biol Regul Homeost Agents, doi:10.23812/CONTI-E?utm_medium=email&utm_source=transaction
Dagan, Barda, Kepten, BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting, N Engl J Med, doi:10.1056/NEJMoa2101765?utm_medium=email&utm_source=transaction
Duan, Liu, Li, Effectiveness of convalescent plasma therapy in severe COVID-19 patients, Proc Natl Acad Sci U S A, doi:10.1073/pnas.2004168117?utm_medium=email&utm_source=transaction
Fauci, Lane, Redfield, Covid-19 -navigating the uncharted, N Engl J Med, doi:10.1056/NEJMe2002387?utm_medium=email&utm_source=transaction
Hansen, Baum, Pascal, Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail, Science, doi:10.1126/science.abd0827?utm_medium=email&utm_source=transaction
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052?utm_medium=email&utm_source=transaction
Joyner, Carter, Senefeld, Convalescent plasma antibody levels and the risk of death from Covid-19, N Engl J Med, doi:10.1056/NEJMoa2031893?utm_medium=email&utm_source=transaction
Levi, Thachil, Iba, Levy, Coagulation abnormalities and thrombosis in patients with COVID-19, Lancet Haematol, doi:10.1016/S2352-3026(20)30145-9?utm_medium=email&utm_source=transaction
Li, Zhang, Hu, Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.10044?utm_medium=email&utm_source=transaction
Marovich, Mascola, Cohen, Monoclonal antibodies for prevention and treatment of COVID-19, JAMA, doi:10.1001/jama.2020.10245?utm_medium=email&utm_source=transaction
Pinto, Park, Beltramello, Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody, Nature, doi:10.1038/s41586-020-2349-y?utm_medium=email&utm_source=transaction
Regeneron, None
Rogers, Shehadeh, Mylona, Convalescent plasma for patients with severe COVID-19: a matched cohort study, Clin Infect Dis, doi:10.1093/cid/ciaa1548?utm_medium=email&utm_source=transaction
Rosas, Bräu, Waters, Tocilizumab in hospitalized patients with COVID-19 pneumonia, MedRxiv, doi:10.1101/2020.08.27.20183442?utm_medium=email&utm_source=transaction
Shang, Zhao, Hu, Du, Cao, On the use of corticosteroids for 2019-nCoV pneumonia, Lancet, doi:10.1016/S0140-6736(20)30361-5?utm_medium=email&utm_source=transaction
Spinner, Gottlieb, Criner, Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19. A randomized clinical trial, JAMA, doi:10.1001/jama.2020.16349?utm_medium=email&utm_source=transaction
Tang, Li, Wang, Sun, Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia, J Thromb Haemost, doi:10.1111/jth.14768?utm_medium=email&utm_source=transaction
Vabret, Britton, Gruber, Immunology of COVID-19: current state of the science, Immunity, doi:10.1016/j.immuni.2020.05.002?utm_medium=email&utm_source=transaction
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2035002?utm_medium=email&utm_source=transaction
Zhou, Fu, Zheng, Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients, Natl Sci Rev, doi:10.1093/nsr/nwaa041?utm_medium=email&utm_source=transaction
DOI record:
{
"DOI": "10.7759/cureus.14933",
"ISSN": [
"2168-8184"
],
"URL": "http://dx.doi.org/10.7759/cureus.14933",
"author": [
{
"affiliation": [],
"family": "Alam",
"given": "Mohammud M",
"sequence": "first"
},
{
"affiliation": [],
"family": "Mahmud",
"given": "Saborny",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aggarwal",
"given": "Sandeep",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fathma",
"given": "Sawsan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al Mahi",
"given": "Naim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shibli",
"given": "Mohammed S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haque",
"given": "Siddiqi M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahmud",
"given": "Sharothy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmed",
"given": "Ziauddin",
"sequence": "additional"
}
],
"container-title": "Cureus",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
5,
10
]
],
"date-time": "2021-05-10T15:18:59Z",
"timestamp": 1620659939000
},
"deposited": {
"date-parts": [
[
2024,
2,
9
]
],
"date-time": "2024-02-09T19:26:10Z",
"timestamp": 1707506770000
},
"indexed": {
"date-parts": [
[
2024,
2,
10
]
],
"date-time": "2024-02-10T00:06:12Z",
"timestamp": 1707523572364
},
"is-referenced-by-count": 5,
"issued": {
"date-parts": [
[
2021,
5,
10
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.cureus.com/articles/58640-clinical-impact-of-the-early-use-of-monoclonal-antibody-ly-cov555-bamlanivimab-on-mortality-and-hospitalization-among-elderly-nursing-home-patients-a-multicenter-retrospective-study",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.7759",
"published": {
"date-parts": [
[
2021,
5,
10
]
]
},
"published-print": {
"date-parts": [
[
2021,
5,
10
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1056/NEJMe2002387",
"article-title": "Covid-19 - navigating the uncharted",
"author": "Fauci AS",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref1",
"unstructured": "Fauci AS, Lane HC, Redfield RR. Covid-19 - navigating the uncharted. N Engl J Med. 2020, 382:1268-9. 10.1056/NEJMe2002387",
"volume": "382",
"year": "2020"
},
{
"key": "ref2",
"unstructured": "COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. (2020). Accessed. April 5, 2021: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6."
},
{
"key": "ref3",
"unstructured": "The New York Times. How many of these 68,000 deaths could have been avoided?. (2020). https.//www.nytimes.com/2020/09/05/opinion/sunday/coronavirus-nursing-homes-deaths.html."
},
{
"DOI": "10.1001/jamanetworkopen.2020.8857",
"article-title": "Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial",
"author": "Borba MGS",
"doi-asserted-by": "publisher",
"journal-title": "JAMA Netw Open",
"key": "ref4",
"unstructured": "Borba MGS, Val FFA, Sampaio VS, et al.. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020, 3:e208857. 10.1001/jamanetworkopen.2020.8857",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.16349",
"article-title": "Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19. A randomized clinical trial",
"author": "Spinner CD",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "ref5",
"unstructured": "Spinner CD, Gottlieb RL, Criner GJ, et al.. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19. A randomized clinical trial. JAMA. 2020, 324:1048-57. 10.1001/jama.2020.16349",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.11.191",
"article-title": "A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness",
"author": "Ahmed S",
"doi-asserted-by": "publisher",
"journal-title": "Int J Infect Dis",
"key": "ref6",
"unstructured": "Ahmed S, Karim MM, Ross AG, et al.. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021, 103:214-6. 10.1016/j.ijid.2020.11.191",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1101/2020.08.27.20183442",
"article-title": "Tocilizumab in hospitalized patients with COVID-19 pneumonia [Preprint]",
"author": "Rosas IO",
"doi-asserted-by": "publisher",
"journal-title": "MedRxiv",
"key": "ref7",
"unstructured": "Rosas IO, Bräu N, Waters M, et al.. Tocilizumab in hospitalized patients with COVID-19 pneumonia [Preprint]. MedRxiv. 2020, 10.1101/2020.08.27.20183442",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30361-5",
"article-title": "On the use of corticosteroids for 2019-nCoV pneumonia",
"author": "Shang L",
"doi-asserted-by": "publisher",
"journal-title": "Lancet",
"key": "ref8",
"unstructured": "Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020, 395:683-4. 10.1016/S0140-6736(20)30361-5",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.10044",
"article-title": "Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial",
"author": "Li L",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "ref9",
"unstructured": "Li L, Zhang W, Hu Y, et al.. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020, 324:460-7. 10.1001/jama.2020.10044",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1101/2020.05.18.20066902",
"article-title": "Doxycycline and hydroxychloroquine as treatment for high-risk COVID-19 patients: experience from case series of 54 patients in long-term care facilities [Preprint]",
"author": "Ahmad I",
"doi-asserted-by": "publisher",
"journal-title": "MedRxiv",
"key": "ref10",
"unstructured": "Ahmad I, Alam M, Saadi R, et al.. Doxycycline and hydroxychloroquine as treatment for high-risk COVID-19 patients: experience from case series of 54 patients in long-term care facilities [Preprint]. MedRxiv. 2020, 10.1101/2020.05.18.20066902",
"year": "2020"
},
{
"DOI": "10.7759/cureus.9658",
"article-title": "Clinical outcomes of early treatment with doxycycline for 89 high-risk COVID-19 patients in long-term care facilities in New York",
"author": "Alam MM",
"doi-asserted-by": "publisher",
"journal-title": "Cureus",
"key": "ref11",
"unstructured": "Alam MM, Mahmud S, Rahman MM, Simpson J, Aggarwal S, Ahmed Z. Clinical outcomes of early treatment with doxycycline for 89 high-risk COVID-19 patients in long-term care facilities in New York. Cureus. 2020, 12:e9658. 10.7759/cureus.9658",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann M",
"doi-asserted-by": "publisher",
"journal-title": "Cell",
"key": "ref12",
"unstructured": "Hoffmann M, Kleine-Weber H, Schroeder S, et al.. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020, 181:271-280.e8. 10.1016/j.cell.2020.02.052",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1126/science.abd0827",
"article-title": "Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail",
"author": "Hansen J",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "ref13",
"unstructured": "Hansen J, Baum A, Pascal KE, et al.. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020, 369:1010-4. 10.1126/science.abd0827",
"volume": "369",
"year": "2020"
},
{
"key": "ref14",
"unstructured": "Regeneron. (2020). https.//investor.regeneron.com/news-releases/news-release-details/regenerons-covid-19-outpatient-trial-prospectively-...."
},
{
"DOI": "10.1056/NEJMoa2029849",
"article-title": "SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19",
"author": "Chen P",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref15",
"unstructured": "Chen P, Nirula A, Heller B, et al.. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021, 384:229-37. 10.1056/NEJMoa2029849",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19",
"author": "Weinreich DM",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref16",
"unstructured": "Weinreich DM, Sivapalasingam S, Norton T, et al.. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021, 384:238-51. 10.1056/NEJMoa2035002",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/j.immuni.2020.05.002",
"article-title": "Immunology of COVID-19: current state of the science",
"author": "Vabret N",
"doi-asserted-by": "publisher",
"journal-title": "Immunity",
"key": "ref17",
"unstructured": "Vabret N, Britton GJ, Gruber C, et al.. Immunology of COVID-19: current state of the science. Immunity. 2020, 52:910-41. 10.1016/j.immuni.2020.05.002",
"volume": "52",
"year": "2020"
},
{
"DOI": "10.1093/nsr/nwaa041",
"article-title": "Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients",
"author": "Zhou Y",
"doi-asserted-by": "publisher",
"journal-title": "Natl Sci Rev",
"key": "ref18",
"unstructured": "Zhou Y, Fu B, Zheng X, et al.. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. 2020, 7:nwaa041. 10.1093/nsr/nwaa041",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.23812/CONTI-E",
"article-title": "Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COV-19 or SARS-CoV-2): anti-inflammatory strategies",
"author": "Conti P",
"doi-asserted-by": "publisher",
"journal-title": "J Biol Regul Homeost Agents",
"key": "ref19",
"unstructured": "Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, Kritas SK. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COV-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020, 34:327-31. 10.23812/CONTI-E",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1016/S2352-3026(20)30145-9",
"article-title": "Coagulation abnormalities and thrombosis in patients with COVID-19",
"author": "Levi M",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Haematol",
"key": "ref20",
"unstructured": "Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7:e438-40. 10.1016/S2352-3026(20)30145-9",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1111/jth.14768",
"article-title": "Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia",
"author": "Tang N",
"doi-asserted-by": "publisher",
"journal-title": "J Thromb Haemost",
"key": "ref21",
"unstructured": "Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020, 18:844-7. 10.1111/jth.14768",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2028436",
"article-title": "Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults",
"author": "Anderson EJ",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref22",
"unstructured": "Anderson EJ, Rouphael NG, Widge AT, et al.. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020, 383:2427-38. 10.1056/NEJMoa2028436",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2101765",
"article-title": "BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting",
"author": "Dagan N",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref23",
"unstructured": "Dagan N, Barda N, Kepten E, et al.. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021, 384:1412-23. 10.1056/NEJMoa2101765",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1548",
"article-title": "Convalescent plasma for patients with severe COVID-19: a matched cohort study",
"author": "Rogers R",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis",
"key": "ref24",
"unstructured": "Rogers R, Shehadeh F, Mylona EK, et al.. Convalescent plasma for patients with severe COVID-19: a matched cohort study. Clin Infect Dis. 2020, ciaa1548. 10.1093/cid/ciaa1548",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2004168117",
"article-title": "Effectiveness of convalescent plasma therapy in severe COVID-19 patients",
"author": "Duan K",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "ref25",
"unstructured": "Duan K, Liu B, Li C, et al.. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020, 117:9490-6. 10.1073/pnas.2004168117",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2031893",
"article-title": "Convalescent plasma antibody levels and the risk of death from Covid-19",
"author": "Joyner MJ",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref26",
"unstructured": "Joyner MJ, Carter RE, Senefeld JW, et al.. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med. 2021, 384:1015-27. 10.1056/NEJMoa2031893",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2349-y",
"article-title": "Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody",
"author": "Pinto D",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "ref27",
"unstructured": "Pinto D, Park YJ, Beltramello M, et al.. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020, 583:290-5. 10.1038/s41586-020-2349-y",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.10245",
"article-title": "Monoclonal antibodies for prevention and treatment of COVID-19",
"author": "Marovich M",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "ref28",
"unstructured": "Marovich M, Mascola JR, Cohen MS. Monoclonal antibodies for prevention and treatment of COVID-19. JAMA. 2020, 324:131-2. 10.1001/jama.2020.10245",
"volume": "324",
"year": "2020"
},
{
"key": "ref29",
"unstructured": "U.S. Food and Drug Administration. Coronavirus (COVID-19) update. FDA authorizes monoclonal antibody for treatment of COVID-19. (2020). Accessed: November 10, 2020: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-tr...."
},
{
"key": "ref30",
"unstructured": "U.S. Food and Drug Administration. Coronavirus (COVID-19) update. FDA authorizes monoclonal antibody for treatment of COVID-19. (2021). Accessed: April 17, 2021: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorizati...."
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.cureus.com/articles/58640-clinical-impact-of-the-early-use-of-monoclonal-antibody-ly-cov555-bamlanivimab-on-mortality-and-hospitalization-among-elderly-nursing-home-patients-a-multicenter-retrospective-study"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Aerospace Engineering"
],
"subtitle": [],
"title": "Clinical Impact of the Early Use of Monoclonal Antibody LY-CoV555 (Bamlanivimab) on Mortality and Hospitalization Among Elderly Nursing Home Patients: A Multicenter Retrospective Study",
"type": "journal-article"
}
