Lack of effectiveness of Bebtelovimab monoclonal antibody among high-risk patients with SARS-Cov-2 Omicron during BA.2, BA.2.12.1 and BA.5 subvariants dominated era
et al., PLOS ONE, doi:10.1371/journal.pone.0279326, Apr 2023
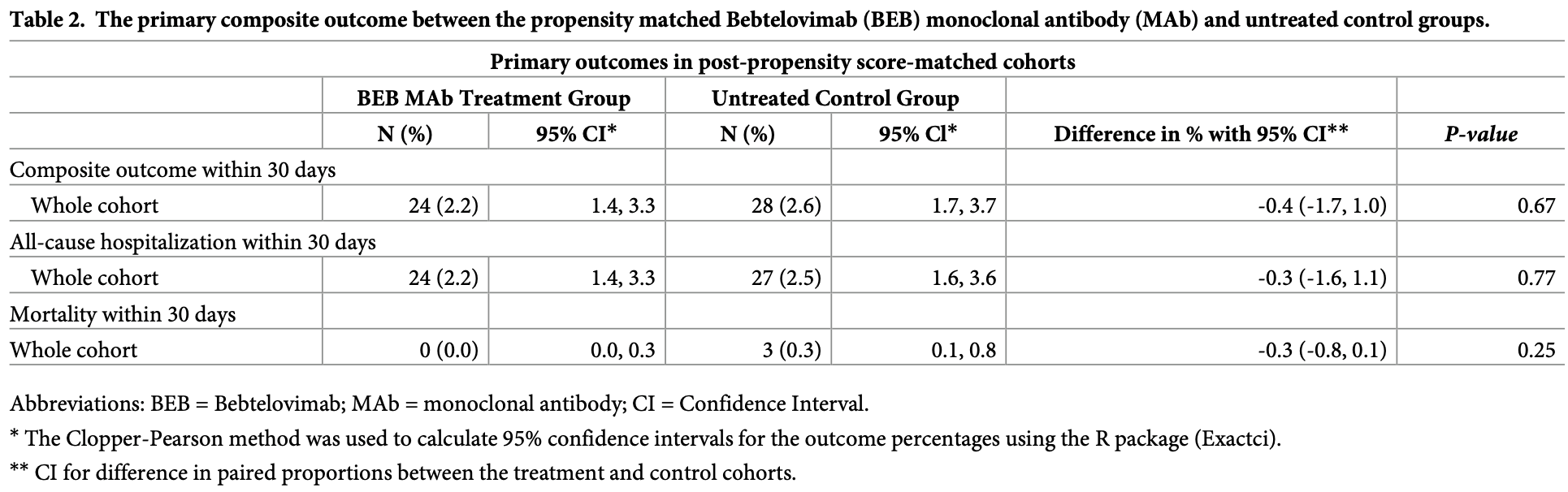
PSM retrospective 19,778 high-risk outpatients in the USA, showing no significant difference in outcomes with bebtelovimab treatment.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending bebtelovimab also recommended them, or
because the patient seeking out bebtelovimab is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Therefore, these kind of studies may
overestimate efficacy.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BQ.1.14, BA.5, BA.2.75, XBB5,6, XBB.1.5, XBB.1.9.16.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments7.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 85.7% lower, RR 0.14, p = 0.25, treatment 0 of 1,091 (0.0%), control 3 of 1,091 (0.3%), NNT 364, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), propensity score matching, day 30.
|
|
risk of death/hospitalization, 25.0% lower, HR 0.75, p = 0.31, treatment 24 of 1,091 (2.2%), control 28 of 1,091 (2.6%), adjusted per study, propensity score matching, multivariable, Cox proportional hazards.
|
|
risk of hospitalization, 11.1% lower, RR 0.89, p = 0.78, treatment 24 of 1,091 (2.2%), control 27 of 1,091 (2.5%), NNT 364, propensity score matching, day 30RETRO.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
5.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Sridhara et al., 28 Apr 2023, retrospective, USA, peer-reviewed, 13 authors, study period 5 April, 2022 - 1 August, 2022.
Contact: btanriover@arizona.edu.
Lack of effectiveness of Bebtelovimab monoclonal antibody among high-risk patients with SARS-Cov-2 Omicron during BA.2, BA.2.12.1 and BA.5 subvariants dominated era
PLOS ONE, doi:10.1371/journal.pone.0279326
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron subvariants are expected to be resistant to Bebtelovimab (BEB) monoclonal antibody (MAb) and the realworld experience regarding its effectiveness is scarce. This retrospective cohort study reports a data analysis in Banner Healthcare System (a large not-for-profit organization) between 4/5/2022 and 8/1/2022 and included 19,778 Coronavirus disease-19 (COVID-19) positive (by PCR or direct antigen testing) patients who were selected from Cerner-Electronic Health Record after the exclusions criteria were met. The study index date for cohort was determined as the date of BEB MAb administration or the date of the first positive COVID-19 testing. The cohort consist of COVID-19 infected patients who received BEB MAb (N = 1,091) compared to propensity score (PS) matched control (N = 1,091). The primary composite outcome was the incidence of 30-day all-cause hospitalization and/or mortality. All statistical analyses were conducted on the paired (matched) dataset. For the primary composite outcome, the event counts and percentages were reported. Ninety-five percent Clopper-Pearson confidence intervals for percentages were computed. The study cohorts were 1:1 propensity matched without replacement across 26 covariates using an optimal matching algorithm that minimizes the sum of absolute pairwise distance across the matched sample after fitting and using logistic regression as the distance function. The pairs were matched exactly on patient vaccination status, BMI group, age group and diabetes status. Compared to the PS matched control group (2.6%; 95% confidence interval [CI]: 1.7%, 3.7%), BEB MAb use (2.2%; 95% CI: 1.4%, 3.3%) did not significantly reduce the incidence
Writing -original draft: Srilekha Sridhara, Ahmet B. Gungor, Halil K. Erol, Mohanad Al-Obaidi, Tirdad T. Zangeneh, Venkatesh K. Ariyamuthu, Aneesha Shetty, Abd A. Qannus, Katherine Mendoza, Sangeetha Murugapandian, Gaurav Gupta, Bekir Tanriover.
References
Adjei, Hong, Molinari, Bull-Otterson, Ajani et al., Mortality Risk Among Patients Hospitalized Primarily for COVID-19 During the Omicron and Delta Variant Pandemic Periods -United States, April 2020, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7137a4
Asian, None
Austin, An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies, Multivariate Behav Res, doi:10.1080/00273171.2011.568786
Ben, Klopfer, Optimal Full Matching and Related Designs via Network Flows, Journal of Computational and Graphical Statistics, doi:10.1198/106186006X137047
Bruel, Hadjadj, Maes, Planas, Seve et al., Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies, Nat Med, doi:10.1038/s41591-022-01792-5
Cao, Jian, Wang, Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution, doi:10.1038/s41586-022-05644-7
Cao, Yisimayi, Jian, Song, Xiao et al., 2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection, Nature, doi:10.1038/s41586-022-04980-y
Covariants, None
Dougan, Azizad, Chen, alone or together with bamlanivimab and etesevimab, as a broadly neutralizing monoclonal antibody treatment for mild to moderate, ambulatory COVID-19, doi:10.1101/2022.03.10.22272100
Esper, Adhikari, Tu, Cheng, El-Haddad et al., Alpha to Omicron: Disease Severity and Clinical Outcomes of Major SARS-CoV-2 Variants, J Infect Dis, doi:10.1093/infdis/jiac411
Gershengorn, Patel, Ferreira, Das, Parekh et al., The clinical effectiveness of REGEN-COV in SARS-CoV-2 infection with Omicron versus Delta variants, PLoS One, doi:10.1371/journal.pone.0278770
Iketani, Liu, Guo, Liu, Chan et al., Antibody evasion properties of SARS-CoV-2 Omicron sublineages, Nature, doi:10.1038/s41586-022-04594-4
Ishak, Mehendale, Alrawashdeh, Sestacovschi, Sharath et al., The association of COVID-19 severity and susceptibility and genetic risk factors: A systematic review of the literature, Gene, doi:10.1016/j.gene.2022.146674
Kousathanas, Pairo-Castineira, Rawlik, Stuckey, Odhams et al., Whole-genome sequencing reveals host factors underlying critical COVID-19, Nature, doi:10.1038/s41586-022-04576-6
Mccreary, Kip, Collins, Minnier, Snyder et al., Evaluation of Bebtelovimab for Treatment of Covid-19 During the SARS-CoV-2 Omicron Variant Era, Open Forum Infect Dis, doi:10.1093/ofid/ofac517
Menni, Valdes, Polidori, Antonelli, Penamakuri et al., Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study, Lancet, doi:10.1016/S0140-6736%2822%2900327-0
Niemi, Daly, Ganna, The human genetic epidemiology of COVID-19, Nat Rev Genet, doi:10.1038/s41576-022-00478-5
Razonable Rr O'horo, Hanson, Arndt, Speicher, Seville, Comparable Outcomes for Bebtelovimab and Ritonavir-Boosted Nirmatrelvir Treatment in High-Risk Patients With Coronavirus Disease-2019 During Severe Acute Respiratory Syndrome Coronavirus 2 BA.2 Omicron Epoch, J Infect Dis, doi:10.1093/infdis/jiac346
Shertel, Lange, Salerno, Hedvat, Jennings et al., Bebtelovimab for Treatment of COVID-19 in Ambulatory Solid Organ Transplant Recipients, Transplantation, doi:10.1097/TP.0000000000004278
Stokes, Zambrano, Anderson, Marder, Raz et al., Coronavirus Disease 2019 Case Surveillance-United States, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm6924e2
Takashita, Yamayoshi, Simon, Van Bakel, Sordillo et al., Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants, N Engl J Med, doi:10.1056/NEJMc2207519
Westendorf, Zentelis, Wang, Foster, Vaillancourt et al., LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants, Cell Rep, doi:10.1016/j.celrep.2022.110812
Wu, Mcgoogan, Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention, JAMA
Yetmar, Beam, Horo, Seville, Brumble et al., Outcomes of bebtelovimab and sotrovimab treatment of solid organ transplant recipients with mild-to-moderate coronavirus disease 2019 during the Omicron epoch, Transpl Infect Dis, doi:10.1111/tid.13901
Zeberg, Paabo, The major genetic risk factor for severe COVID-19 is inherited from Neanderthals, Nature, doi:10.1038/s41586-020-2818-3
DOI record:
{
"DOI": "10.1371/journal.pone.0279326",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0279326",
"abstract": "<jats:p>Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron subvariants are expected to be resistant to Bebtelovimab (BEB) monoclonal antibody (MAb) and the real-world experience regarding its effectiveness is scarce. This retrospective cohort study reports a data analysis in Banner Healthcare System (a large not-for-profit organization) between 4/5/2022 and 8/1/2022 and included 19,778 Coronavirus disease-19 (COVID-19) positive (by PCR or direct antigen testing) patients who were selected from Cerner-Electronic Health Record after the exclusions criteria were met. The study index date for cohort was determined as the date of BEB MAb administration or the date of the first positive COVID-19 testing. The cohort consist of COVID-19 infected patients who received BEB MAb (N = 1,091) compared to propensity score (PS) matched control (N = 1,091). The primary composite outcome was the incidence of 30-day all-cause hospitalization and/or mortality. All statistical analyses were conducted on the paired (matched) dataset. For the primary composite outcome, the event counts and percentages were reported. Ninety-five percent Clopper-Pearson confidence intervals for percentages were computed. The study cohorts were 1:1 propensity matched without replacement across 26 covariates using an optimal matching algorithm that minimizes the sum of absolute pairwise distance across the matched sample after fitting and using logistic regression as the distance function. The pairs were matched exactly on patient vaccination status, BMI group, age group and diabetes status. Compared to the PS matched control group (2.6%; 95% confidence interval [CI]: 1.7%, 3.7%), BEB MAb use (2.2%; 95% CI: 1.4%, 3.3%) did not significantly reduce the incidence of the primary outcome (p = 0.67). In the subgroup analysis, we observed similar no-difference trends regarding the primary outcomes for the propensity rematched BEB MAb treated and untreated groups, stratified by patient vaccination status, age (<65 years or ≥65), and immunocompromised status (patients with HIV/AIDS or solid organ transplants or malignancy including lymphoproliferative disorder). The number needed to treat (1/0.026–0.022) with BEB MAb was 250 to avoid one hospitalization and/or death over 30 days. The BEB MAb use lacked efficacy in patients with SARS-CoV-2 Omicron subvariants (mainly BA.2, BA.2.12.1, and BA.5) in the Banner Healthcare System in the Southwestern United States.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Sridhara",
"given": "Srilekha",
"sequence": "first"
},
{
"affiliation": [],
"family": "Gungor",
"given": "Ahmet B.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5760-4276",
"affiliation": [],
"authenticated-orcid": true,
"family": "Erol",
"given": "Halil K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al-Obaidi",
"given": "Mohanad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zangeneh",
"given": "Tirdad T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bedrick",
"given": "Edward J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ariyamuthu",
"given": "Venkatesh K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shetty",
"given": "Aneesha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qannus",
"given": "Abd A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9618-2336",
"affiliation": [],
"authenticated-orcid": true,
"family": "Mendoza",
"given": "Katherine",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9145-5283",
"affiliation": [],
"authenticated-orcid": true,
"family": "Murugapandian",
"given": "Sangeetha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gupta",
"given": "Gaurav",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2378-9302",
"affiliation": [],
"authenticated-orcid": true,
"family": "Tanriover",
"given": "Bekir",
"sequence": "additional"
}
],
"container-title": "PLOS ONE",
"container-title-short": "PLoS ONE",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2023,
4,
28
]
],
"date-time": "2023-04-28T17:25:19Z",
"timestamp": 1682702719000
},
"deposited": {
"date-parts": [
[
2023,
4,
28
]
],
"date-time": "2023-04-28T17:25:51Z",
"timestamp": 1682702751000
},
"editor": [
{
"affiliation": [],
"family": "Molla",
"given": "Md Maruf Ahmed",
"sequence": "first"
}
],
"funder": [
{
"name": "Shionogi Inc. and La Jolla pharmaceuticals"
},
{
"DOI": "10.13039/501100019123",
"doi-asserted-by": "publisher",
"name": "AiCuris"
}
],
"indexed": {
"date-parts": [
[
2023,
4,
29
]
],
"date-time": "2023-04-29T04:34:58Z",
"timestamp": 1682742898480
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2023,
4,
28
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2023,
4,
28
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
4,
28
]
],
"date-time": "2023-04-28T00:00:00Z",
"timestamp": 1682640000000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0279326",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0279326",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2023,
4,
28
]
]
},
"published-online": {
"date-parts": [
[
2023,
4,
28
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention.",
"author": "Z Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"issue": "13",
"journal-title": "JAMA",
"key": "pone.0279326.ref001",
"volume": "323",
"year": "2020"
},
{
"key": "pone.0279326.ref002",
"unstructured": "CDC COVID19 Symptoms. Available on https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed on 2/25/2023."
},
{
"DOI": "10.15585/mmwr.mm6924e2",
"article-title": "Coronavirus Disease 2019 Case Surveillance—United States, January 22-May 30, 2020.",
"author": "EK Stokes",
"doi-asserted-by": "crossref",
"first-page": "759",
"issue": "24",
"journal-title": "MMWR Morb Mortal Wkly Rep.",
"key": "pone.0279326.ref003",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(22)00327-0",
"article-title": "Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study",
"author": "C Menni",
"doi-asserted-by": "crossref",
"first-page": "1618",
"issue": "10335",
"journal-title": "Lancet",
"key": "pone.0279326.ref004",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiac411",
"article-title": "Alpha to Omicron: Disease Severity and Clinical Outcomes of Major SARS-CoV-2 Variants",
"author": "FP Esper",
"doi-asserted-by": "crossref",
"first-page": "344",
"issue": "3",
"journal-title": "J Infect Dis",
"key": "pone.0279326.ref005",
"volume": "227",
"year": "2023"
},
{
"DOI": "10.1038/s41586-022-04594-4",
"article-title": "Antibody evasion properties of SARS-CoV-2 Omicron sublineages",
"author": "S Iketani",
"doi-asserted-by": "crossref",
"first-page": "553",
"issue": "7906",
"journal-title": "Nature",
"key": "pone.0279326.ref006",
"volume": "604",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2207519",
"article-title": "Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants",
"author": "E Takashita",
"doi-asserted-by": "crossref",
"first-page": "468",
"issue": "5",
"journal-title": "N Engl J Med",
"key": "pone.0279326.ref007",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1038/s41591-022-01792-5",
"article-title": "Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies",
"author": "T Bruel",
"doi-asserted-by": "crossref",
"first-page": "1297",
"issue": "6",
"journal-title": "Nat Med",
"key": "pone.0279326.ref008",
"volume": "28",
"year": "2022"
},
{
"key": "pone.0279326.ref009",
"unstructured": "US Food and Drug Administration. COVID-19 update: FDA authorizes new monoclonal antibody for treatment of COVID-19 that retains activity against Omicron variant. Available at: https://www.fda.gov/news-events/ press-announcements/coronavirus-covid-19-update-fdaauthorizes-new-monoclonal-antibody-treatment-covid-19-retains. Accessed on November 11, 2022."
},
{
"DOI": "10.1371/journal.pone.0278770",
"article-title": "The clinical effectiveness of REGEN-COV in SARS-CoV-2 infection with Omicron versus Delta variants.",
"author": "HB Gershengorn",
"doi-asserted-by": "crossref",
"first-page": "e0278770",
"issue": "12",
"journal-title": "PLoS One",
"key": "pone.0279326.ref010",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1101/2022.03.10.22272100",
"doi-asserted-by": "crossref",
"key": "pone.0279326.ref011",
"unstructured": "Dougan M, Azizad M, Chen P, et al. Bebtelovimab, alone or together with bamlanivimab and etesevimab, as a broadly neutralizing monoclonal antibody treatment for mild to moderate, ambulatory COVID-19. medRxiv. Preprint and has not been peer-reviewed. Available at https://doi.org/10.1101/2022.03.10.22272100."
},
{
"key": "pone.0279326.ref012",
"unstructured": "CDC COVID19 Underlying Medical Conditions. Available on https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. Accessed on 2/25/2023."
},
{
"DOI": "10.1093/ofid/ofac517",
"article-title": "Evaluation of Bebtelovimab for Treatment of Covid-19 During the SARS-CoV-2 Omicron Variant Era.",
"author": "EK McCreary",
"doi-asserted-by": "crossref",
"first-page": "ofac517",
"issue": "10",
"journal-title": "Open Forum Infect Dis.",
"key": "pone.0279326.ref013",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiac346",
"article-title": "Comparable Outcomes for Bebtelovimab and Ritonavir-Boosted Nirmatrelvir Treatment in High-Risk Patients With Coronavirus Disease-2019 During Severe Acute Respiratory Syndrome Coronavirus 2 BA.2 Omicron Epoch",
"author": "O Razonable RR",
"doi-asserted-by": "crossref",
"first-page": "1683",
"issue": "10",
"journal-title": "J Infect Dis",
"key": "pone.0279326.ref014",
"volume": "226",
"year": "2022"
},
{
"key": "pone.0279326.ref015",
"unstructured": "CoVariants. Available at https://covariants.org/, accessed on 11/10/2022."
},
{
"DOI": "10.1080/00273171.2011.568786",
"article-title": "An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies.",
"author": "PC Austin",
"doi-asserted-by": "crossref",
"first-page": "399",
"issue": "3",
"journal-title": "Multivariate Behav Res.",
"key": "pone.0279326.ref016",
"volume": "46",
"year": "2011"
},
{
"DOI": "10.1198/106186006X137047",
"article-title": "Optimal Full Matching and Related Designs via Network Flows",
"author": "Ben B Hansen",
"doi-asserted-by": "crossref",
"first-page": "609",
"issue": "3",
"journal-title": "Journal of Computational and Graphical Statistics",
"key": "pone.0279326.ref017",
"volume": "15",
"year": "2006"
},
{
"key": "pone.0279326.ref018",
"unstructured": "Arizona State Immunization Information System [ASIIS], available at https://asiis.azdhs.gov/, accessed on 11/12/2022."
},
{
"DOI": "10.15585/mmwr.mm7137a4",
"article-title": "Mortality Risk Among Patients Hospitalized Primarily for COVID-19 During the Omicron and Delta Variant Pandemic Periods—United States, April 2020-June 2022.",
"author": "S Adjei",
"doi-asserted-by": "crossref",
"first-page": "1182",
"issue": "37",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "pone.0279326.ref019",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04980-y",
"article-title": "BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection",
"author": "Y Cao",
"doi-asserted-by": "crossref",
"first-page": "593",
"issue": "7923",
"journal-title": "Nature",
"key": "pone.0279326.ref020",
"volume": "608",
"year": "2022"
},
{
"DOI": "10.1016/j.celrep.2022.110812",
"article-title": "LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants.",
"author": "K Westendorf",
"doi-asserted-by": "crossref",
"first-page": "110812",
"issue": "7",
"journal-title": "Cell Rep",
"key": "pone.0279326.ref021",
"volume": "39",
"year": "2022"
},
{
"DOI": "10.1097/TP.0000000000004278",
"article-title": "Bebtelovimab for Treatment of COVID-19 in Ambulatory Solid Organ Transplant Recipients",
"author": "T Shertel",
"doi-asserted-by": "crossref",
"first-page": "e463",
"issue": "10",
"journal-title": "Transplantation",
"key": "pone.0279326.ref022",
"volume": "106",
"year": "2022"
},
{
"DOI": "10.1111/tid.13901",
"article-title": "Outcomes of bebtelovimab and sotrovimab treatment of solid organ transplant recipients with mild-to-moderate coronavirus disease 2019 during the Omicron epoch.",
"author": "ZA Yetmar",
"doi-asserted-by": "crossref",
"first-page": "e13901",
"issue": "4",
"journal-title": "Transpl Infect Dis",
"key": "pone.0279326.ref023",
"volume": "24",
"year": "2022"
},
{
"key": "pone.0279326.ref024",
"unstructured": "The NIH COVID-19 Treatment Guidelines Panel’s Statement on Omicron Subvariants, Pre-Exposure Prophylaxis, and Therapeutic Management of Nonhospitalized Patients With COVID-19. Available at: https://www.covid19treatmentguidelines.nih.gov/therapies/statement-on-omicron-subvariants/. Accessed on November 20, 2022."
},
{
"article-title": "Fact sheet for healthcare providers: Emergency Use Authorization for bebtelovimab.",
"author": "Food and Drug Administration",
"key": "pone.0279326.ref025",
"year": "2022"
},
{
"article-title": "Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution.",
"author": "Y Cao",
"journal-title": "bioRxiv",
"key": "pone.0279326.ref026",
"year": "2022"
},
{
"key": "pone.0279326.ref027",
"unstructured": "FDA Announces Bebtelovimab is Not Currently Authorized in Any US Region. Avaiable at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-bebtelovimab-not-currently-authorized-any-us-region. Accessed on December 3, 2022."
},
{
"DOI": "10.1038/s41576-022-00478-5",
"article-title": "The human genetic epidemiology of COVID-19",
"author": "MEK Niemi",
"doi-asserted-by": "crossref",
"first-page": "533",
"issue": "9",
"journal-title": "Nat Rev Genet",
"key": "pone.0279326.ref028",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1038/s41586-020-2818-3",
"article-title": "The major genetic risk factor for severe COVID-19 is inherited from Neanderthals",
"author": "H Zeberg",
"doi-asserted-by": "crossref",
"first-page": "610",
"issue": "7835",
"journal-title": "Nature",
"key": "pone.0279326.ref029",
"volume": "587",
"year": "2020"
},
{
"DOI": "10.1016/j.gene.2022.146674",
"article-title": "The association of COVID-19 severity and susceptibility and genetic risk factors: A systematic review of the literature",
"author": "A Ishak",
"doi-asserted-by": "crossref",
"first-page": "146674",
"journal-title": "Gene",
"key": "pone.0279326.ref030",
"volume": "836",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04576-6",
"article-title": "Whole-genome sequencing reveals host factors underlying critical COVID-19",
"author": "A Kousathanas",
"doi-asserted-by": "crossref",
"first-page": "97",
"issue": "7917",
"journal-title": "Nature",
"key": "pone.0279326.ref031",
"volume": "607",
"year": "2022"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pone.0279326"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Lack of effectiveness of Bebtelovimab monoclonal antibody among high-risk patients with SARS-Cov-2 Omicron during BA.2, BA.2.12.1 and BA.5 subvariants dominated era",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pone.corrections_policy",
"volume": "18"
}