The clinical effectiveness of REGEN-COV in SARS-CoV-2 infection with Omicron versus Delta variants
et al., PLOS ONE, doi:10.1371/journal.pone.0278770, Dec 2022
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 2,083 outpatients in the USA, showing higher risk of hospitalization with casirivimab/imdevimab, without statistical significance. There may be significant unadjusted confounding by indication.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in the after exclusion results of meta-analysis:
substantial unadjusted confounding by indication possible.
|
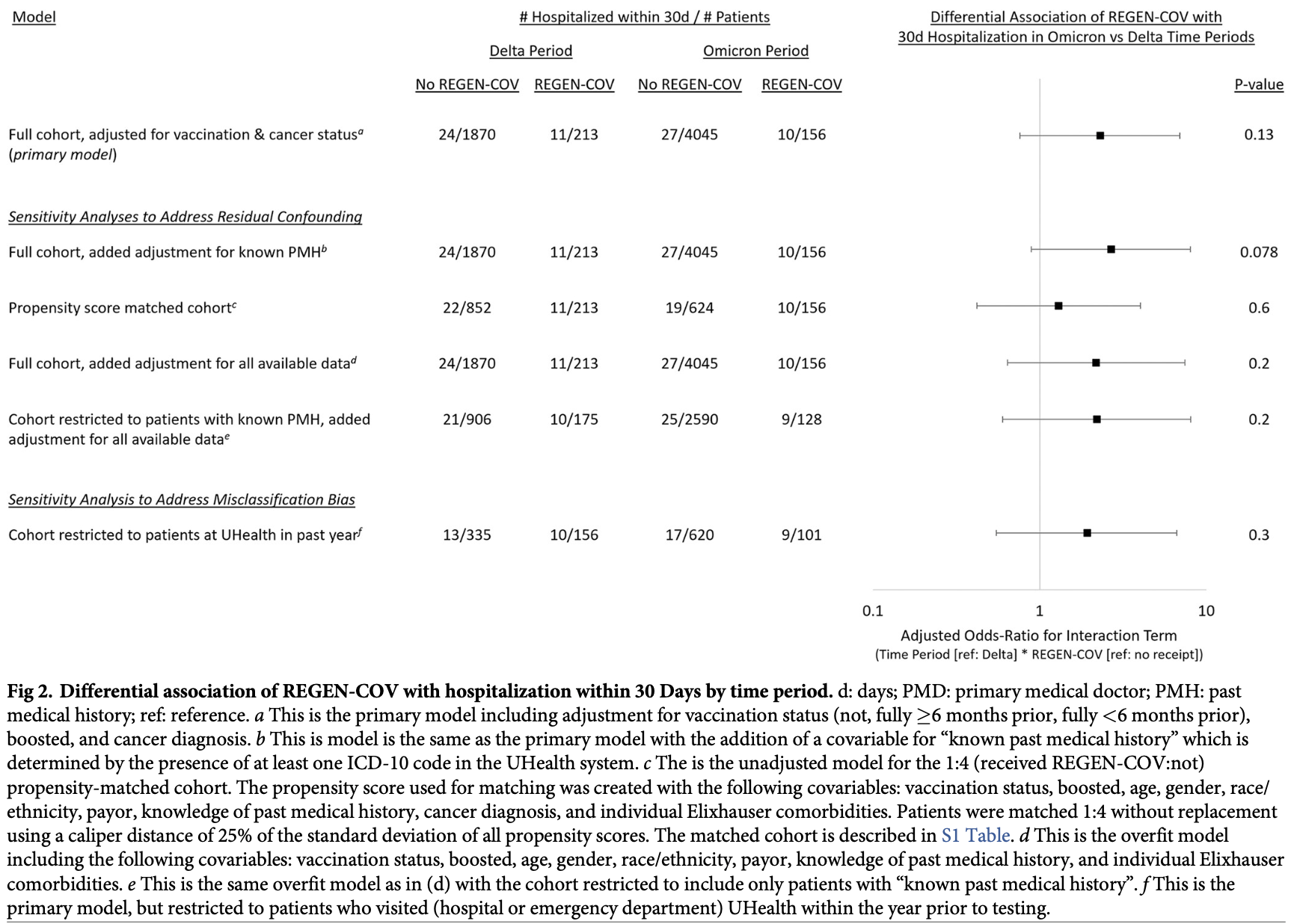
risk of hospitalization, 95.0% higher, OR 1.95, p = 0.09, treatment 369, control 5,915, adjusted per study, multivariable, day 30, RR approximated with OR.
|
|
risk of hospitalization, 104.9% higher, RR 2.05, p = 0.009, treatment 21 of 369 (5.7%), control 41 of 1,476 (2.8%), propensity score matching, day 30, Figure 2, PSM cohort.
|
|
risk of hospitalization, 100% higher, RR 2.00, p = 0.07, treatment 11 of 213 (5.2%), control 22 of 852 (2.6%), delta, propensity score matching, day 30, Figure 2, PSM cohort.
|
|
risk of hospitalization, 110.5% higher, RR 2.11, p = 0.06, treatment 10 of 156 (6.4%), control 19 of 624 (3.0%), omicron, propensity score matching, day 30, Figure 2, PSM cohort.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Gershengorn et al., 2 Dec 2022, retrospective, USA, peer-reviewed, 6 authors.
Contact: hbg20@med.miami.edu, hsro@miami.edu.
The clinical effectiveness of REGEN-COV in SARS-CoV-2 infection with Omicron versus Delta variants
PLOS ONE, doi:10.1371/journal.pone.0278770
Background In vitro studies suggesting that REGEN-COV (casirivimab plus imdevimab monoclonal antibodies) had poor efficacy against Omicron-variant SARS-CoV-2 infection led to amendment of REGEN-COV's Emergency Use Authorization to recommend use only in regions without high Omicron prevalence. REGEN-COV's relative clinical effectiveness for Omicron is unknown.
Methods and findings We conducted a retrospective cohort study of non-hospitalized adults who tested positive for SARS-CoV-2 by polymerase chain reaction at the
Supporting information
S1 Table .
References
Brehm, Pfefferle, Possel, Karolyi, Zoufaly et al., Clinical efficacy and in vitro neutralization capacity of monoclonal antibodies for SARS-CoV-2 delta and omicron variants, J Med Virol, doi:10.1002/jmv.27916
Cao, Wang, Jian, Song, Yisimayi, Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, Nature, doi:10.1038/s41586-021-04385-3
Chen, Winkler, Case, Aziati, Bricker et al., In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains, Nature, doi:10.1038/s41586-021-03720-y
Deyo, Cherkin, Ciol, Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases, J Clin Epidemiol, doi:10.1016/0895-4356%2892%2990133-8
Falcone, Tiseo, Valoriani, Barbieri, Occhineri et al., Efficacy of Bamlanivimab/ Etesevimab and Casirivimab/Imdevimab in Preventing Progression to Severe COVID-19 and Role of Variants of Concern, Infect Dis Ther, doi:10.1007/s40121-021-00525-4
Hachmann, Miller, Collier, Ventura, Yu et al., Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5, N Engl J Med, doi:10.1056/NEJMc2206576
Keating, Dong, Meko, Visualizing the omicron wave striking and rolling across the country, The Washington Post
Lusvarghi, Pollett, Neerukonda, Wang, Vassell, SARS-CoV-2 BA.1 variant is neutralized by vaccine booster-elicited serum but evades most convalescent serum and therapeutic antibodies, Sci Transl Med, doi:10.1126/scitranslmed.abn8543
Shaughnessy, Emergency Use Authorization 091
Tao, Tzou, Pond, Ioannidis, Shafer, Susceptibility of SARS-CoV-2 Omicron Variants to Therapeutic Monoclonal Antibodies: Systematic Review and Meta-analysis, Microbiol Spectr, doi:10.1128/spectrum.00926-22
Vanblargan, Errico, Halfmann, Zost, Crowe et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies, Nat Med, doi:10.1038/s41591-021-01678-y
Ward, Bermingham, Ayoubkhani, Gethings, Pouwels et al., Risk of covid-19 related deaths for SARS-CoV-2 omicron (B.1.1.529) compared with delta (B.1.617.2): retrospective cohort study, BMJ, doi:10.1136/bmj-2022-070695
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2108163
DOI record:
{
"DOI": "10.1371/journal.pone.0278770",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0278770",
"abstract": "<jats:sec id=\"sec001\">\n<jats:title>Background</jats:title>\n<jats:p>In vitro studies suggesting that REGEN-COV (casirivimab plus imdevimab monoclonal antibodies) had poor efficacy against Omicron-variant SARS-CoV-2 infection led to amendment of REGEN-COV’s Emergency Use Authorization to recommend use only in regions without high Omicron prevalence. REGEN-COV’s relative clinical effectiveness for Omicron is unknown.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec002\">\n<jats:title>Methods and findings</jats:title>\n<jats:p>We conducted a retrospective cohort study of non-hospitalized adults who tested positive for SARS-CoV-2 by polymerase chain reaction at the University of Miami Health System from July 19 –November 21, 2021 (Delta period) and December 6, 2021 –January 7, 2022 (Omicron period). Subjects were stratified be REGEN-COV receipt within 72h of test positivity and by time period of infection. We constructed multivariable logistic regression models to assess the differential association of REGEN-COV receipt with hospitalization within 30 days (primary outcome) and ED presentation; all models included three exposure terms (REGEN-COV receipt, Omicron vs Delta period, interaction of REGEN-COV with time period) and potential confounders (vaccination status, vaccine boosting, cancer diagnosis). Our cohort consisted of 2,083 adults in the Delta period (213 [10.2%] received REGEN-COV) and 4,201 in the Omicron period (156 [3.7%] received REGEN-COV). Hospitalization was less common during the Omicron period than during Delta (0.9% vs 1.7%, p = 0.78) and more common for patients receiving REGEN-COV than not (5.7% vs 0.9%, p<0.001). After adjustment, we found no differential association of REGEN-COV use during Omicron vs Delta with hospitalization within 30d (adjusted odds ratio [95% confidence interval] for the interaction term: 2.31 [0.76–6.92], p = 0.13). Similarly, we found no differential association for hospitalization within 15d (2.45 [0.63–9.59], p = 0.20) or emergency department presentation within 30d (1.43 [0.57–3.51], p = 0.40) or within 15d (1.79 [0.65–4.82], p = 0.30).</jats:p>\n</jats:sec>\n<jats:sec id=\"sec003\">\n<jats:title>Conclusions</jats:title>\n<jats:p>Within the limitations of this study’s power to detect a difference, we identified no differential effectiveness of REGEN-COV in the context of Omicron vs Delta SARS-CoV-2 infection.</jats:p>\n</jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7360-2489",
"affiliation": [],
"authenticated-orcid": true,
"family": "Gershengorn",
"given": "Hayley B.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Patel",
"given": "Samira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferreira",
"given": "Tanira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Das",
"given": "Sankalp",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parekh",
"given": "Dipen J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shukla",
"given": "Bhavarth",
"sequence": "additional"
}
],
"container-title": "PLOS ONE",
"container-title-short": "PLoS ONE",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2022,
12,
2
]
],
"date-time": "2022-12-02T18:25:55Z",
"timestamp": 1670005555000
},
"deposited": {
"date-parts": [
[
2022,
12,
2
]
],
"date-time": "2022-12-02T18:26:17Z",
"timestamp": 1670005577000
},
"editor": [
{
"affiliation": [],
"family": "Thanachartwet",
"given": "Vipa",
"sequence": "first"
}
],
"funder": [
{
"name": "University of Miami Hospital and Clinics Data Analytics Research Team"
},
{
"name": "University of Miami Hospital and Clinics Data Analytics Research Team"
},
{
"name": "University of Miami Hospital and Clinics Data Analytics Research Team"
},
{
"name": "University of Miami Hospital and Clinics Data Analytics Research Team"
},
{
"name": "University of Miami Hospital and Clinics Data Analytics Research Team"
},
{
"name": "University of Miami Hospital and Clinics Data Analytics Research Team"
}
],
"indexed": {
"date-parts": [
[
2022,
12,
3
]
],
"date-time": "2022-12-03T06:03:37Z",
"timestamp": 1670047417289
},
"is-referenced-by-count": 0,
"issue": "12",
"issued": {
"date-parts": [
[
2022,
12,
2
]
]
},
"journal-issue": {
"issue": "12",
"published-online": {
"date-parts": [
[
2022,
12,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
2
]
],
"date-time": "2022-12-02T00:00:00Z",
"timestamp": 1669939200000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0278770",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0278770",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2022,
12,
2
]
]
},
"published-online": {
"date-parts": [
[
2022,
12,
2
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"author": "JA O’Shaughnessy",
"key": "pone.0278770.ref001",
"volume-title": "Emergency Use Authorization 091",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2108163",
"article-title": "REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19",
"author": "DM Weinreich",
"doi-asserted-by": "crossref",
"first-page": "e81",
"issue": "23",
"journal-title": "N Engl J Med",
"key": "pone.0278770.ref002",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1126/scitranslmed.abn8543",
"article-title": "SARS-CoV-2 BA.1 variant is neutralized by vaccine booster-elicited serum but evades most convalescent serum and therapeutic antibodies",
"author": "S Lusvarghi",
"doi-asserted-by": "crossref",
"first-page": "eabn8543",
"issue": "645",
"journal-title": "Sci Transl Med",
"key": "pone.0278770.ref003",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1038/s41591-021-01678-y",
"article-title": "An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies",
"author": "LA VanBlargan",
"doi-asserted-by": "crossref",
"first-page": "490",
"issue": "3",
"journal-title": "Nat Med",
"key": "pone.0278770.ref004",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-04385-3",
"article-title": "Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies",
"author": "Y Cao",
"doi-asserted-by": "crossref",
"first-page": "657",
"issue": "7898",
"journal-title": "Nature",
"key": "pone.0278770.ref005",
"volume": "602",
"year": "2022"
},
{
"article-title": "Clinical efficacy and in vitro neutralization capacity of monoclonal antibodies for SARS-CoV-2 delta and omicron variants",
"author": "TT Brehm",
"journal-title": "J Med Virol",
"key": "pone.0278770.ref006",
"year": "2022"
},
{
"DOI": "10.1128/spectrum.00926-22",
"article-title": "Susceptibility of SARS-CoV-2 Omicron Variants to Therapeutic Monoclonal Antibodies: Systematic Review and Meta-analysis.",
"author": "K Tao",
"doi-asserted-by": "crossref",
"first-page": "e0092622",
"journal-title": "Microbiol Spectr.",
"key": "pone.0278770.ref007",
"year": "2022"
},
{
"article-title": "Visualizing the omicron wave striking and rolling across the country.",
"author": "D Keating",
"first-page": "2022",
"journal-title": "The Washington Post.",
"key": "pone.0278770.ref008",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/0895-4356(92)90133-8",
"article-title": "Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases.",
"author": "RA Deyo",
"doi-asserted-by": "crossref",
"first-page": "613",
"issue": "6",
"journal-title": "J Clin Epidemiol",
"key": "pone.0278770.ref009",
"volume": "45",
"year": "1992"
},
{
"DOI": "10.1038/s41586-021-03720-y",
"article-title": "In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains",
"author": "RE Chen",
"doi-asserted-by": "crossref",
"first-page": "103",
"issue": "7870",
"journal-title": "Nature",
"key": "pone.0278770.ref010",
"volume": "596",
"year": "2021"
},
{
"DOI": "10.1007/s40121-021-00525-4",
"article-title": "Efficacy of Bamlanivimab/Etesevimab and Casirivimab/Imdevimab in Preventing Progression to Severe COVID-19 and Role of Variants of Concern.",
"author": "M Falcone",
"doi-asserted-by": "crossref",
"first-page": "2479",
"issue": "4",
"journal-title": "Infect Dis Ther.",
"key": "pone.0278770.ref011",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1136/bmj-2022-070695",
"article-title": "Risk of covid-19 related deaths for SARS-CoV-2 omicron (B.1.1.529) compared with delta (B.1.617.2): retrospective cohort study.",
"author": "IL Ward",
"doi-asserted-by": "crossref",
"first-page": "e070695",
"journal-title": "BMJ",
"key": "pone.0278770.ref012",
"volume": "378",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2206576",
"article-title": "Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5",
"author": "NP Hachmann",
"doi-asserted-by": "crossref",
"first-page": "86",
"issue": "1",
"journal-title": "N Engl J Med",
"key": "pone.0278770.ref013",
"volume": "387",
"year": "2022"
}
],
"reference-count": 13,
"references-count": 13,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pone.0278770"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "The clinical effectiveness of REGEN-COV in SARS-CoV-2 infection with Omicron versus Delta variants",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pone.corrections_policy",
"volume": "17"
}
