A Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19
, NEJM, doi:10.1056/NEJMoa2033130, ACTIV-3, NCT04501978, Dec 2020
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Late stage RCT of LY-CoV555 added to remdesivir, showing non-statistically significant higher mortality with the addition of LY-CoV555.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments6.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 100% higher, HR 2.00, p = 0.22, treatment 9 of 163 (5.5%), control 5 of 151 (3.3%), adjusted per study, proportional hazards regression.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
ACTIV-3/TICO LY-CoV555 study group et al., 22 Dec 2020, Randomized Controlled Trial, USA, peer-reviewed, 1 author, study period 5 August, 2020 - 13 October, 2020, average treatment delay 7.0 days, trial NCT04501978 (history) (ACTIV-3).
A Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19
New England Journal of Medicine, doi:10.1056/nejmoa2033130
BACKGROUND LY-CoV555, a neutralizing monoclonal antibody, has been associated with a decrease in viral load and the frequency of hospitalizations or emergency department visits among outpatients with coronavirus disease 2019 (Covid-19). Data are needed on the effect of this antibody in patients who are hospitalized with Covid-19.
METHODS In this platform trial of therapeutic agents, we randomly assigned hospitalized patients who had Covid-19 without end-organ failure in a 1:1 ratio to receive either LY-CoV555 or matching placebo. In addition, all the patients received high-quality supportive care as background therapy, including the antiviral drug remdesivir and, when indicated, supplemental oxygen and glucocorticoids. LY-CoV555 (at a dose of 7000 mg) or placebo was administered as a single intravenous infusion over a 1-hour period. The primary outcome was a sustained recovery during a 90-day period, as assessed in a time-to-event analysis. An interim futility assessment was performed on the basis of a seven-category ordinal scale for pulmonary function on day 5.
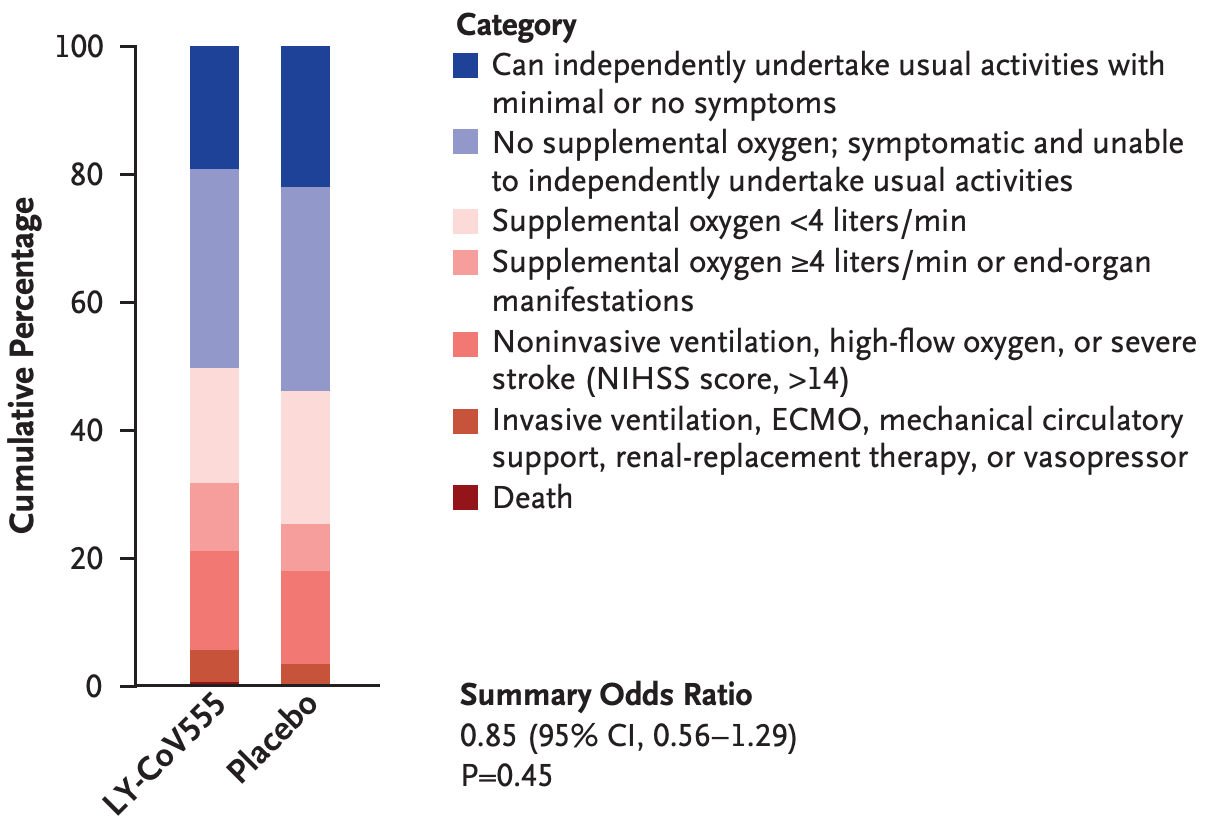
RESULTS On October 26, 2020, the data and safety monitoring board recommended stopping enrollment for futility after 314 patients (163 in the LY-CoV555 group and 151 in the placebo group) had undergone randomization and infusion. The median interval since the onset of symptoms was 7 days (interquartile range, 5 to 9). At day 5, a total of 81 patients (50%) in the LY-CoV555 group and 81 (54%) in the placebo group were in one of the two most favorable categories of the pulmonary outcome. Across the seven categories, the odds ratio of being in a more favorable category in the LY-CoV555 group than in the placebo group was 0.85 (95% confidence interval [CI], 0.56 to 1.29; P = 0.45). The percentage of patients with the primary safety outcome (a composite of death, serious adverse events, or clinical grade 3 or 4 adverse events through day 5) was similar in the LY-CoV555 group and the placebo group (19% and 14%, respectively; odds ratio, 1.56; 95% CI, 0.78 to 3.10; P = 0.20). The rate ratio for a sustained recovery was 1.06 (95% CI, 0.77 to 1.47).
CONCLUSIONS Monoclonal antibody LY-CoV555, when coadministered with remdesivir, did not demonstrate efficacy among hospitalized patients who had Covid-19 without end-organ failure. (Funded by Operation Warp Speed and others; TICO ClinicalTrials.gov number, NCT04501978.
References
Arvin, Fink, Schmid, A perspective on potential antibodydependent enhancement of SARS-CoV-2, Nature
Baum, Fulton, Wloga, Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies, Science
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19 -final report, N Engl J Med
Chen, Nirula, Heller, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2029849
Core, R: a language and environment for statistical computing
Jones, Brown-Augsburger, Corbett, LY-CoV555, a rapidly isolated potent neutralizing antibody, provides protection in a non-human primate model of SARS-CoV-2 infection, doi:10.1101/2020.09.30.318972v3
Lee, Wheatley, Kent, Dekosky, Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies, Nat Microbiol
Mccullagh, Regression models for ordinal data, J R Stat Soc B
Mulangu, Dodd, Rt, A randomized, controlled trial of Ebola virus disease therapeutics, N Engl J Med
Murray, Babiker, Baker, Design and implementation of the multi-arm, multi-stage Therapeutics for Inpatients with COVID-19 (TICO) platform master protocol: an Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) initiative, doi:10.1101/2020.11.08.20227876v2
The, Group, Dexamethasone in hospitalized patients with Covid-19 -preliminary report, N Engl J Med, doi:10.1056/NEJMoa2021436
Weisblum, Schmidt, Zhang, Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants, doi:10.1101/2020.07.21.214759v1
DOI record:
{
"DOI": "10.1056/nejmoa2033130",
"ISSN": [
"0028-4793",
"1533-4406"
],
"URL": "http://dx.doi.org/10.1056/NEJMoa2033130",
"alternative-id": [
"10.1056/NEJMoa2033130"
],
"author": [
{
"affiliation": [],
"name": "ACTIV-3/TICO LY-CoV555 Study Group",
"sequence": "first"
}
],
"container-title": "New England Journal of Medicine",
"container-title-short": "N Engl J Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
12,
22
]
],
"date-time": "2020-12-22T22:00:26Z",
"timestamp": 1608674426000
},
"deposited": {
"date-parts": [
[
2023,
5,
5
]
],
"date-time": "2023-05-05T17:54:00Z",
"timestamp": 1683309240000
},
"funder": [
{
"DOI": "10.13039/100000060",
"doi-asserted-by": "publisher",
"name": "NIAID"
},
{
"DOI": "10.13039/100015624",
"doi-asserted-by": "crossref",
"name": "Leidos"
},
{
"DOI": "10.13039/100000050",
"doi-asserted-by": "publisher",
"name": "National Heart, Lung, and Blood Institute"
},
{
"DOI": "10.13039/100000738",
"doi-asserted-by": "publisher",
"name": "U.S. Department of Veterans Affairs"
},
{
"award": [
"grant no. 126"
],
"name": "National Research Foundation, Denmark"
},
{
"DOI": "10.13039/501100000925",
"doi-asserted-by": "publisher",
"name": "National Health and Medical Research Council"
},
{
"DOI": "10.13039/501100000265",
"award": [
"MRC_UU_12023/23"
],
"doi-asserted-by": "crossref",
"name": "Medical Research Council, UK"
},
{
"name": "Operation Warp Speed"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
6
]
],
"date-time": "2024-04-06T07:51:24Z",
"timestamp": 1712389884935
},
"is-referenced-by-count": 319,
"issue": "10",
"issued": {
"date-parts": [
[
2021,
3,
11
]
]
},
"journal-issue": {
"issue": "10",
"published-print": {
"date-parts": [
[
2021,
3,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://www.nejmgroup.org/legal/terms-of-use.htm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
3,
11
]
],
"date-time": "2021-03-11T00:00:00Z",
"timestamp": 1615420800000
}
}
],
"link": [
{
"URL": "http://www.nejm.org/doi/pdf/10.1056/NEJMoa2033130",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "150",
"original-title": [],
"page": "905-914",
"prefix": "10.1056",
"published": {
"date-parts": [
[
2021,
3,
11
]
]
},
"published-print": {
"date-parts": [
[
2021,
3,
11
]
]
},
"publisher": "Massachusetts Medical Society",
"reference": [
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "r1"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "r2"
},
{
"DOI": "10.1056/NEJMoa2029849",
"doi-asserted-by": "publisher",
"key": "r3"
},
{
"author": "McCullagh P",
"first-page": "109",
"journal-title": "J R Stat Soc B",
"key": "r9",
"volume": "42",
"year": "1980"
},
{
"key": "r10",
"unstructured": "R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2020."
},
{
"DOI": "10.1126/science.abd0831",
"doi-asserted-by": "publisher",
"key": "r11"
},
{
"DOI": "10.1038/s41586-020-2538-8",
"doi-asserted-by": "publisher",
"key": "r13"
},
{
"DOI": "10.1038/s41564-020-00789-5",
"doi-asserted-by": "publisher",
"key": "r14"
},
{
"DOI": "10.1056/NEJMoa1910993",
"doi-asserted-by": "publisher",
"key": "r15"
}
],
"reference-count": 9,
"references-count": 9,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.nejm.org/doi/10.1056/NEJMoa2033130"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "A Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19",
"type": "journal-article",
"volume": "384"
}
