Comparative effectiveness of Paxlovid versus sotrovimab and molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients: observational cohort study using the OpenSAFELY platform
et al., medRxiv, doi:10.1101/2023.01.20.23284849, Jan 2023
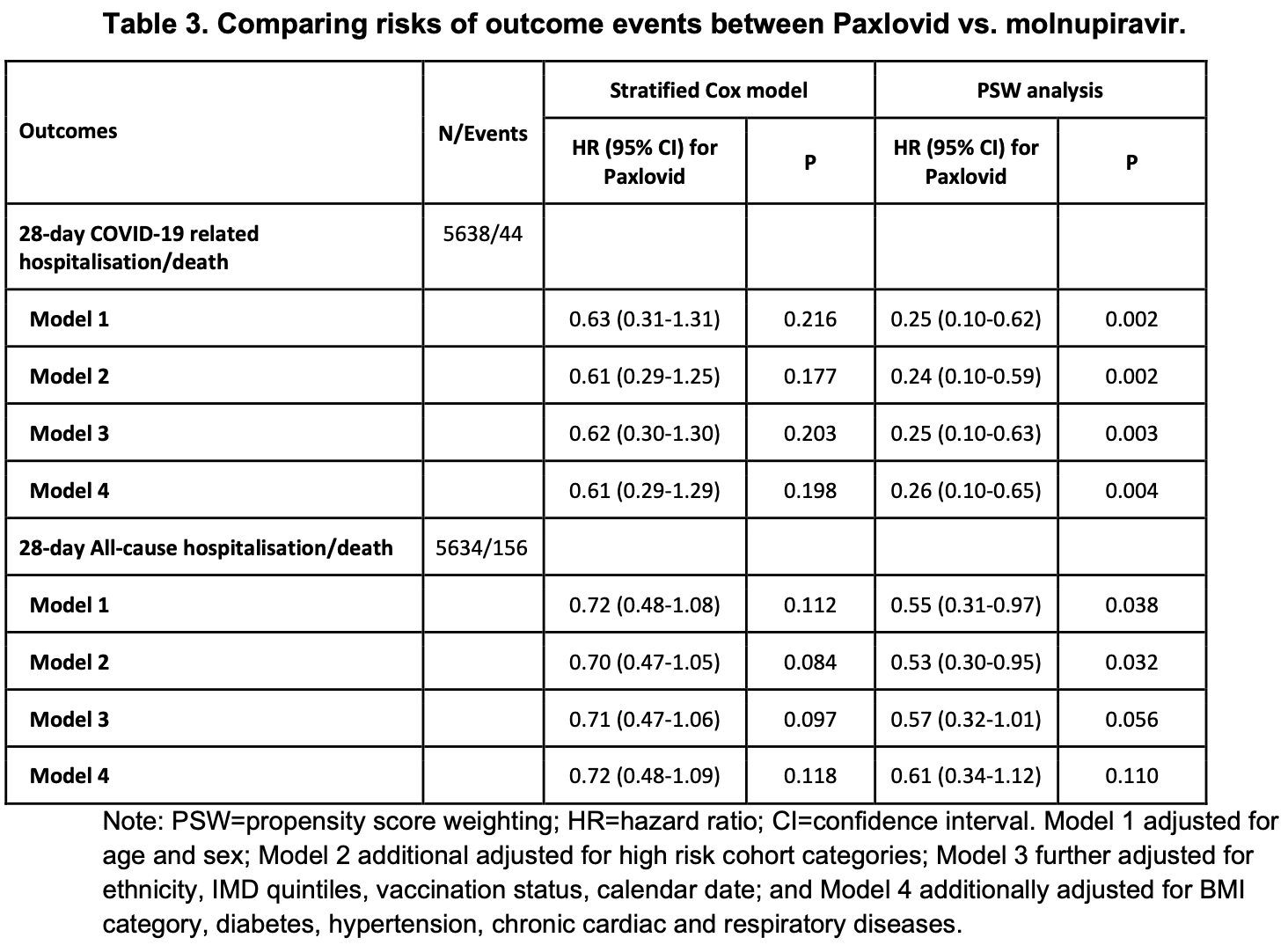
OpenSAFELY retrospective 5,638 outpatients in the UK, showing significantly higher hospitalization/death for molnupiravir compared with paxlovid.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments25.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in the after exclusion results of meta-analysis:
study compares against another treatment showing significant efficacy.
Study covers molnupiravir, sotrovimab, and paxlovid.
|
risk of death/hospitalization, 284.6% higher, HR 3.85, p = 0.004, treatment 11 of 802 (1.4%), control 33 of 4,836 (0.7%), inverted to make HR<1 favor treatment, COVID-19 related, propensity score weighting, Cox proportional hazards, day 28, model 4.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
23.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Zheng et al., 22 Jan 2023, retrospective, United Kingdom, preprint, 9 authors, study period 11 February, 2022 - 1 October, 2022, this trial compares with another treatment - results may be better when compared to placebo.
Contact: laurie.tomlinson@lshtm.ac.uk.
Comparative effectiveness of Paxlovid versus sotrovimab and molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients: observational cohort study using the OpenSAFELY platform
doi:10.1101/2023.01.20.23284849
Objective: To compare the effectiveness of Paxlovid vs. sotrovimab and molnupiravir in preventing severe COVID-19 outcomes in non-hospitalised high-risk COVID-19 adult patients. Design: With the approval of NHS England, we conducted a real-world cohort study using the OpenSAFELY-TPP platform. Setting: Patient-level electronic health record data were obtained from 24 million people registered with a general practice in England that uses TPP software. The primary care data were securely linked with data on COVID-19 infection and therapeutics, hospital admission, and death within the OpenSAFELY-TPP platform, covering a period where both Paxlovid and sotrovimab were first-line treatment options in community settings. Participants: Non-hospitalised adult COVID-19 patients at high risk of severe outcomes treated
Summary In routine care of non-hospitalised high-risk adult patients with COVID-19 in England, we observed no substantial difference in the risk of severe COVID-19 outcomes between those who received Paxlovid and sotrovimab during a period of Omicron BA.2 and BA.5 dominance. .
Administrative
Conflicts of Interest BG has received research funding from the Laura and John Arnold Foundation, the NHS National Institute for Health Research (NIHR), the NIHR School of Primary Care Research, NHS England, the NIHR Oxford Biomedical Research Centre, the Mohn-Westlake Foundation, NIHR Applied Research Collaboration Oxford and Thames Valley, the Wellcome Trust, the Good Thinking Foundation, Health Data Research UK, the Health Foundation, the World Health Organisation, UKRI MRC, Asthma UK, the British Lung Foundation, and the Longitudinal Health and Wellbeing strand of the National Core Studies programme; he is a Non-Executive Director at NHS Digital; he also receives personal income from speaking and writing for lay audiences on the misuse of science. BMK is also employed by NHS England working on medicines policy and clinical lead for primary care medicines data.
References
Agarwal, Rochwerg, Lamontagne, A living WHO guideline on drugs for covid-19, BMJ
Akinosoglou, Schinas, Gogos, Oral Antiviral Treatment for COVID-19: A Comprehensive Review on Nirmatrelvir/Ritonavir, Viruses
Arbel, Sagy, Hoshen, Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge, N Engl J Med
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Butler, Hobbs, Gbinigie, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet
Dryden-Peterson, Kim, Kim, Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System: A Population-Based Cohort Study, Ann Intern Med
Green, Curtis, Higgins, Trends, variation, and clinical characteristics of recipients of antiviral drugs and neutralising monoclonal antibodies for covid-19 in community settings: retrospective, descriptive cohort study of 23.4 million people in OpenSAFELY, BMJ Medicine
Gupta, Gonzalez-Rojas, Juarez, Early Treatment for Covid-19 with SARS-CoV-2
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Nhs, Antivirals or Neutralising Antibodies for Non-Hospitalised Patients with COVID-19
Nhs, Neutralising monoclonal antibodies (nMABs) or antivirals for non-hospitalised patients with COVID-19
Stürmer, Rothman, Avorn, Glynn, Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution--a simulation study, Am J Epidemiol
Stürmer, Webster-Clark, Lund, Propensity Score Weighting and Trimming Strategies for Reducing Variance and Bias of Treatment Effect Estimates: A Simulation Study, Am J Epidemiol
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study, Lancet
Wu, Carr, Harvey, WHO's Therapeutics and COVID-19 Living Guideline on mAbs needs to be reassessed, Lancet
Zheng, Green, Tazare, Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform, BMJ
DOI record:
{
"DOI": "10.1101/2023.01.20.23284849",
"URL": "http://dx.doi.org/10.1101/2023.01.20.23284849",
"abstract": "<jats:p>Objective: To compare the effectiveness of Paxlovid vs. sotrovimab and molnupiravir in preventing severe COVID-19 outcomes in non-hospitalised high-risk COVID-19 adult patients. Design: With the approval of NHS England, we conducted a real-world cohort study using the OpenSAFELY-TPP platform. Setting: Patient-level electronic health record data were obtained from 24 million people registered with a general practice in England that uses TPP software. The primary care data were securely linked with data on COVID-19 infection and therapeutics, hospital admission, and death within the OpenSAFELY-TPP platform, covering a period where both Paxlovid and sotrovimab were first-line treatment options in community settings. Participants: Non-hospitalised adult COVID-19 patients at high risk of severe outcomes treated with Paxlovid, sotrovimab or molnupiravir between February 11, 2022 and October 1, 2022. Interventions: Paxlovid, sotrovimab or molnupiravir administered in the community by COVID-19 Medicine Delivery Units. Main outcome measure: COVID-19 related hospitalisation or COVID-19 related death within 28 days after treatment initiation. Results: A total of 7683 eligible patients treated with Paxlovid (n=4836) and sotrovimab (n=2847) were included in the main analysis. The mean age was 54.3 (SD=14.9) years; 64% were female, 93% White and 93% had three or more COVID-19 vaccinations. Within 28 days after treatment initiation, 52 (0.68%) COVID-19 related hospitalisations/deaths were observed (33 (0.68%) treated with Paxlovid and 19 (0.67%) with sotrovimab). Cox proportional hazards model stratified by region showed that after adjusting for demographics, high-risk cohort categories, vaccination status, calendar time, body mass index and other comorbidities, treatment with Paxlovid was associated with a similar risk of outcome event as treatment with sotrovimab (HR=1.14, 95% CI: 0.62 to 2.08; P=0.673). Results from propensity score weighted Cox model also showed comparable risks in these two treatment groups (HR=0.88, 95% CI: 0.45 to 1.71; P=0.700). An exploratory analysis comparing Paxlovid users with 802 molnupiravir users (11 (1.37%) COVID-19 related hospitalisations/deaths) showed some evidence in favour of Paxlovid but with variation in the effect estimates between models (HR ranging from 0.26 to 0.61). Conclusion: In routine care of non-hospitalised high-risk adult patients with COVID-19 in England, no substantial difference in the risk of severe COVID-19 outcomes was observed between those who received Paxlovid and sotrovimab between February and October 2022, when different subvariants of Omicron were dominant.</jats:p>",
"accepted": {
"date-parts": [
[
2023,
1,
22
]
]
},
"author": [
{
"affiliation": [],
"family": "Zheng",
"given": "Bang",
"sequence": "first"
},
{
"affiliation": [],
"family": "Tazare",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nab",
"given": "Linda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mehrkar",
"given": "Amir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "MacKenna",
"given": "Brian",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5127-4728",
"affiliation": [],
"authenticated-orcid": false,
"family": "Goldacre",
"given": "Ben",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Douglas",
"given": "Ian J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tomlinson",
"given": "Laurie",
"sequence": "additional"
},
{
"affiliation": [],
"name": "The OpenSAFELY Collaborative",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
1,
23
]
],
"date-time": "2023-01-23T06:05:17Z",
"timestamp": 1674453917000
},
"deposited": {
"date-parts": [
[
2023,
1,
23
]
],
"date-time": "2023-01-23T06:05:18Z",
"timestamp": 1674453918000
},
"group-title": "Epidemiology",
"indexed": {
"date-parts": [
[
2023,
1,
24
]
],
"date-time": "2023-01-24T05:56:44Z",
"timestamp": 1674539804597
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
1,
22
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2023.01.20.23284849",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2023,
1,
22
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2023,
1,
22
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2023.01.20.23284849"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Comparative effectiveness of Paxlovid versus sotrovimab and molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients: observational cohort study using the OpenSAFELY platform",
"type": "posted-content"
}
zheng5