Comparative effectiveness of Paxlovid versus sotrovimab and molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients: observational cohort study using the OpenSAFELY platform
et al., medRxiv, doi:10.1101/2023.01.20.23284849, Jan 2023
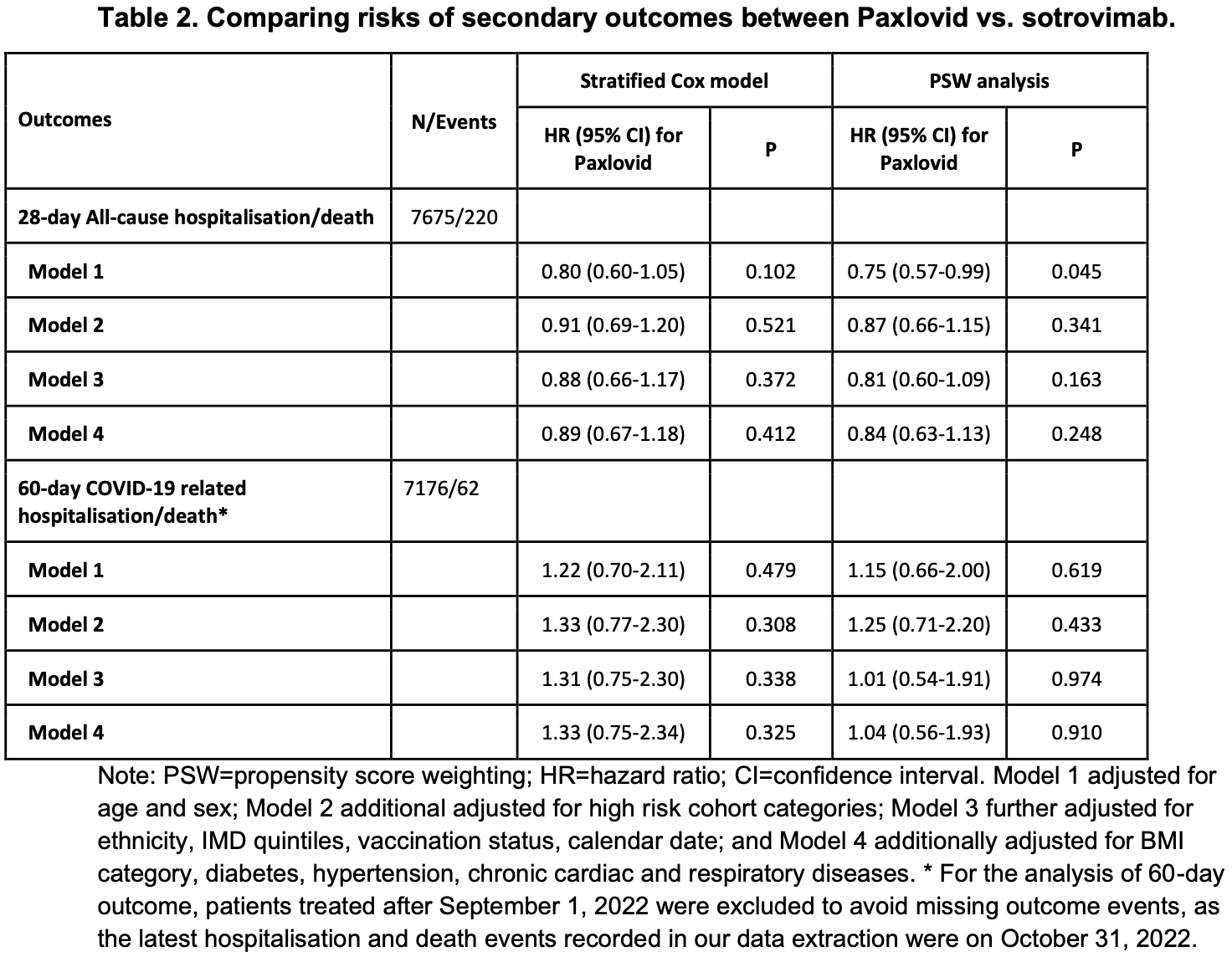
OpenSAFELY retrospective 7,683 outpatients in the UK, showing no significant difference in hospitalization/death between paxlovid and sotrovimab.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments18.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
Study covers molnupiravir, sotrovimab, and paxlovid.
|
risk of death/hospitalization, 4.0% higher, HR 1.04, p = 0.91, treatment 4,836, control 2,847, COVID-19 related, propensity score weighting, Cox proportional hazards, day 60, model 4.
|
|
risk of death/hospitalization, 12.0% lower, HR 0.88, p = 0.70, treatment 33 of 4,836 (0.7%), control 19 of 2,847 (0.7%), COVID-19 related, propensity score weighting, Cox proportional hazards, day 28, model 4.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Zheng et al., 22 Jan 2023, retrospective, United Kingdom, preprint, mean age 54.3, 9 authors, study period 11 February, 2022 - 1 October, 2022, this trial compares with another treatment - results may be better when compared to placebo.
Contact: laurie.tomlinson@lshtm.ac.uk.
Comparative effectiveness of Paxlovid versus sotrovimab and molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients: observational cohort study using the OpenSAFELY platform
doi:10.1101/2023.01.20.23284849
Objective: To compare the effectiveness of Paxlovid vs. sotrovimab and molnupiravir in preventing severe COVID-19 outcomes in non-hospitalised high-risk COVID-19 adult patients. Design: With the approval of NHS England, we conducted a real-world cohort study using the OpenSAFELY-TPP platform. Setting: Patient-level electronic health record data were obtained from 24 million people registered with a general practice in England that uses TPP software. The primary care data were securely linked with data on COVID-19 infection and therapeutics, hospital admission, and death within the OpenSAFELY-TPP platform, covering a period where both Paxlovid and sotrovimab were first-line treatment options in community settings. Participants: Non-hospitalised adult COVID-19 patients at high risk of severe outcomes treated
Summary In routine care of non-hospitalised high-risk adult patients with COVID-19 in England, we observed no substantial difference in the risk of severe COVID-19 outcomes between those who received Paxlovid and sotrovimab during a period of Omicron BA.2 and BA.5 dominance. .
Administrative
Conflicts of Interest BG has received research funding from the Laura and John Arnold Foundation, the NHS National Institute for Health Research (NIHR), the NIHR School of Primary Care Research, NHS England, the NIHR Oxford Biomedical Research Centre, the Mohn-Westlake Foundation, NIHR Applied Research Collaboration Oxford and Thames Valley, the Wellcome Trust, the Good Thinking Foundation, Health Data Research UK, the Health Foundation, the World Health Organisation, UKRI MRC, Asthma UK, the British Lung Foundation, and the Longitudinal Health and Wellbeing strand of the National Core Studies programme; he is a Non-Executive Director at NHS Digital; he also receives personal income from speaking and writing for lay audiences on the misuse of science. BMK is also employed by NHS England working on medicines policy and clinical lead for primary care medicines data.
References
Agarwal, Rochwerg, Lamontagne, A living WHO guideline on drugs for covid-19, BMJ
Akinosoglou, Schinas, Gogos, Oral Antiviral Treatment for COVID-19: A Comprehensive Review on Nirmatrelvir/Ritonavir, Viruses
Arbel, Sagy, Hoshen, Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge, N Engl J Med
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Butler, Hobbs, Gbinigie, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet
Dryden-Peterson, Kim, Kim, Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System: A Population-Based Cohort Study, Ann Intern Med
Green, Curtis, Higgins, Trends, variation, and clinical characteristics of recipients of antiviral drugs and neutralising monoclonal antibodies for covid-19 in community settings: retrospective, descriptive cohort study of 23.4 million people in OpenSAFELY, BMJ Medicine
Gupta, Gonzalez-Rojas, Juarez, Early Treatment for Covid-19 with SARS-CoV-2
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Nhs, Antivirals or Neutralising Antibodies for Non-Hospitalised Patients with COVID-19
Nhs, Neutralising monoclonal antibodies (nMABs) or antivirals for non-hospitalised patients with COVID-19
Stürmer, Rothman, Avorn, Glynn, Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution--a simulation study, Am J Epidemiol
Stürmer, Webster-Clark, Lund, Propensity Score Weighting and Trimming Strategies for Reducing Variance and Bias of Treatment Effect Estimates: A Simulation Study, Am J Epidemiol
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study, Lancet
Wu, Carr, Harvey, WHO's Therapeutics and COVID-19 Living Guideline on mAbs needs to be reassessed, Lancet
Zheng, Green, Tazare, Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform, BMJ
DOI record:
{
"DOI": "10.1101/2023.01.20.23284849",
"URL": "http://dx.doi.org/10.1101/2023.01.20.23284849",
"abstract": "<jats:p>Objective: To compare the effectiveness of Paxlovid vs. sotrovimab and molnupiravir in preventing severe COVID-19 outcomes in non-hospitalised high-risk COVID-19 adult patients. Design: With the approval of NHS England, we conducted a real-world cohort study using the OpenSAFELY-TPP platform. Setting: Patient-level electronic health record data were obtained from 24 million people registered with a general practice in England that uses TPP software. The primary care data were securely linked with data on COVID-19 infection and therapeutics, hospital admission, and death within the OpenSAFELY-TPP platform, covering a period where both Paxlovid and sotrovimab were first-line treatment options in community settings. Participants: Non-hospitalised adult COVID-19 patients at high risk of severe outcomes treated with Paxlovid, sotrovimab or molnupiravir between February 11, 2022 and October 1, 2022. Interventions: Paxlovid, sotrovimab or molnupiravir administered in the community by COVID-19 Medicine Delivery Units. Main outcome measure: COVID-19 related hospitalisation or COVID-19 related death within 28 days after treatment initiation. Results: A total of 7683 eligible patients treated with Paxlovid (n=4836) and sotrovimab (n=2847) were included in the main analysis. The mean age was 54.3 (SD=14.9) years; 64% were female, 93% White and 93% had three or more COVID-19 vaccinations. Within 28 days after treatment initiation, 52 (0.68%) COVID-19 related hospitalisations/deaths were observed (33 (0.68%) treated with Paxlovid and 19 (0.67%) with sotrovimab). Cox proportional hazards model stratified by region showed that after adjusting for demographics, high-risk cohort categories, vaccination status, calendar time, body mass index and other comorbidities, treatment with Paxlovid was associated with a similar risk of outcome event as treatment with sotrovimab (HR=1.14, 95% CI: 0.62 to 2.08; P=0.673). Results from propensity score weighted Cox model also showed comparable risks in these two treatment groups (HR=0.88, 95% CI: 0.45 to 1.71; P=0.700). An exploratory analysis comparing Paxlovid users with 802 molnupiravir users (11 (1.37%) COVID-19 related hospitalisations/deaths) showed some evidence in favour of Paxlovid but with variation in the effect estimates between models (HR ranging from 0.26 to 0.61). Conclusion: In routine care of non-hospitalised high-risk adult patients with COVID-19 in England, no substantial difference in the risk of severe COVID-19 outcomes was observed between those who received Paxlovid and sotrovimab between February and October 2022, when different subvariants of Omicron were dominant.</jats:p>",
"accepted": {
"date-parts": [
[
2023,
1,
22
]
]
},
"author": [
{
"affiliation": [],
"family": "Zheng",
"given": "Bang",
"sequence": "first"
},
{
"affiliation": [],
"family": "Tazare",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nab",
"given": "Linda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mehrkar",
"given": "Amir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "MacKenna",
"given": "Brian",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5127-4728",
"affiliation": [],
"authenticated-orcid": false,
"family": "Goldacre",
"given": "Ben",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Douglas",
"given": "Ian J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tomlinson",
"given": "Laurie",
"sequence": "additional"
},
{
"affiliation": [],
"name": "The OpenSAFELY Collaborative",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
1,
23
]
],
"date-time": "2023-01-23T06:05:17Z",
"timestamp": 1674453917000
},
"deposited": {
"date-parts": [
[
2023,
1,
23
]
],
"date-time": "2023-01-23T06:05:18Z",
"timestamp": 1674453918000
},
"group-title": "Epidemiology",
"indexed": {
"date-parts": [
[
2023,
1,
24
]
],
"date-time": "2023-01-24T05:56:44Z",
"timestamp": 1674539804597
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
1,
22
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2023.01.20.23284849",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2023,
1,
22
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2023,
1,
22
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2023.01.20.23284849"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Comparative effectiveness of Paxlovid versus sotrovimab and molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients: observational cohort study using the OpenSAFELY platform",
"type": "posted-content"
}