Efficacy and Safety of Molnupiravir for the Treatment of Non-Hospitalized Adults With Mild COVID-19: A Randomized, Open-Label, Parallel-Group Phase 3 Trial
et al., SSRN Electronic Journal, doi:10.2139/ssrn.4042673, Feb 2022
RCT 1,220 patients in India, showing lower risk of hospitalization and improved recovery with treatment. CTRI/2021/07/034588.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
|
risk of hospitalization, 46.2% lower, RR 0.54, p = 0.26, treatment 7 of 610 (1.1%), control 13 of 610 (2.1%), NNT 102, day 28.
|
|
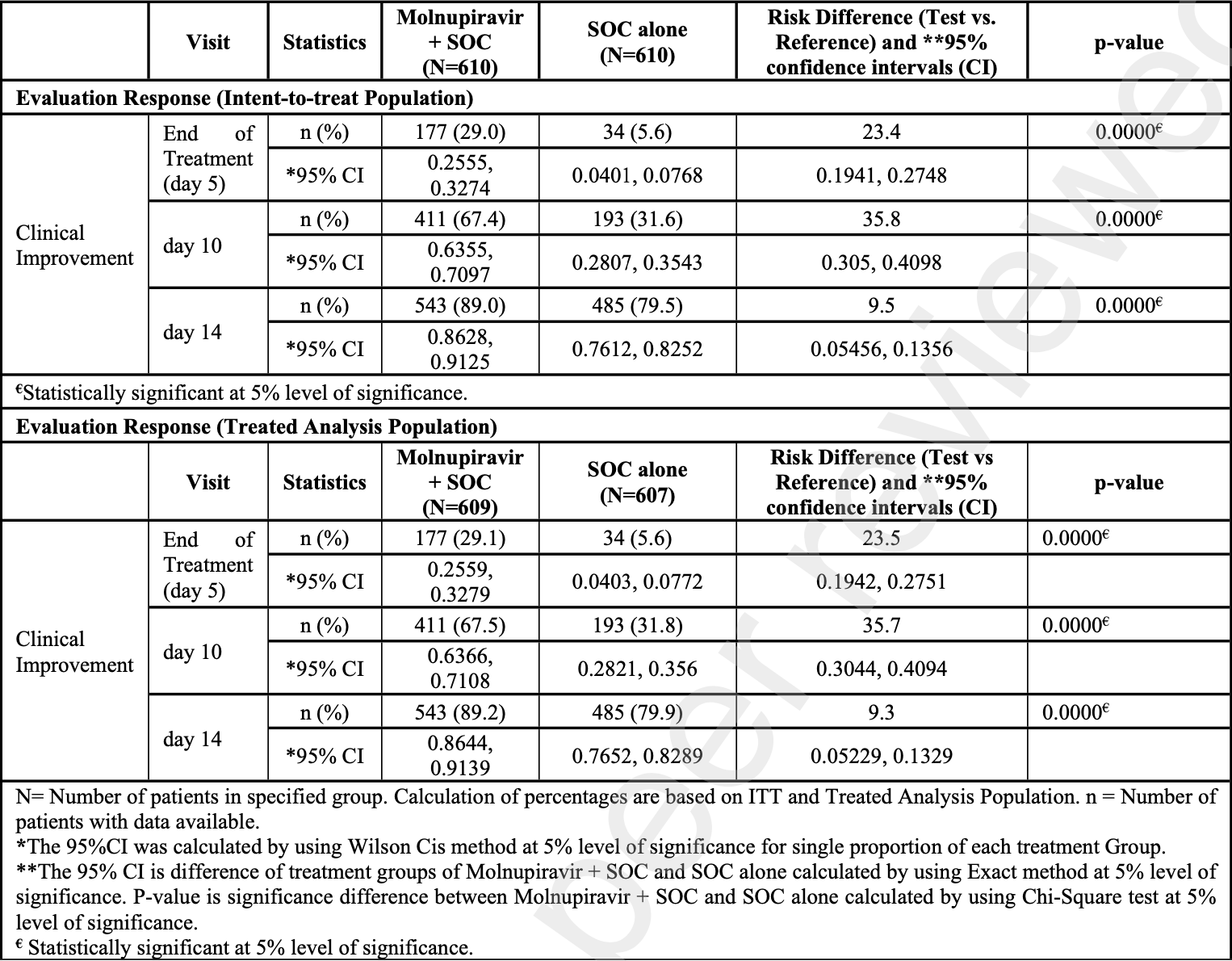
risk of no improvement, 46.4% lower, RR 0.54, p < 0.001, treatment 67 of 610 (11.0%), control 125 of 610 (20.5%), NNT 11, day 14.
|
|
risk of no improvement, 52.3% lower, RR 0.48, p < 0.001, treatment 199 of 610 (32.6%), control 417 of 610 (68.4%), NNT 2.8, day 10.
|
|
risk of no improvement, 24.8% lower, RR 0.75, p < 0.001, treatment 433 of 610 (71.0%), control 576 of 610 (94.4%), NNT 4.3, day 5.
|
|
risk of no viral clearance, 59.2% lower, RR 0.41, p < 0.001, treatment 42 of 610 (6.9%), control 103 of 610 (16.9%), NNT 10.0, day 14.
|
|
risk of no viral clearance, 81.0% lower, RR 0.19, p < 0.001, treatment 62 of 610 (10.2%), control 327 of 610 (53.6%), NNT 2.3, day 10.
|
|
risk of no viral clearance, 77.6% lower, RR 0.22, p < 0.001, treatment 113 of 610 (18.5%), control 505 of 610 (82.8%), NNT 1.6, day 5.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Tippabhotla et al., 24 Feb 2022, Randomized Controlled Trial, India, peer-reviewed, 5 authors, study period 1 July, 2021 - 24 August, 2021, average treatment delay 3.0 days.
Background: Molnupiravir is an oral prodrug with antiviral activity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Molnupiravir was initially developed for Influenza treatment, is being repurposed for the treatment of COVID-19. This study evaluates the efficacy and safety of molnupiravir in addition to the standardof-care (SOC) for the treatment of non-hospitalized patients with mild COVID-19.
Methods: In this phase 3, randomized, open-label, parallel-group study, 1220 patients with laboratory-confirmed (RT-PCR positive) SARS-CoV-2 infection were enrolled across 16 centres in India and 7.3% (90/1220) patients were with one risk factor (i.e. hypertension, diabetes mellitus, obesity, hypothyroidism, hyperthyroidism) presenting a risk for progression to severe COVID-19. Non-hospitalized adults with mild COVID-19 were randomized to receive either molnupiravir 800 mg (200 mg x 4 capsules administered orally every 12 hours for 5 days) with SOC or SOC alone and followed up at day 5 (end of treatment, and days 10, 14 and 28. Standard of care was provided as per the clinical guidance for management of adult COVID-19 patients by the Government of India or as per the Investigator's discretion. The primary endpoint was the rate of hospitalization of patients from randomization till day 14. Secondary endpoints included rate of hospitalization of patients from randomization up to day 28; clinical improvement (2-point decrease in 11-point WHO Clinical Progression Scale) at days 5, 10, and 14; SARS-CoV-2 RT-PCR negativity at the end of treatment; and mortality rate at day 14 and day 28. The study is registered with the Clinical Trials Registry of India, CTRI/2021/07/034588.
References
Businesswire, Merck and Ridgeback Biotherapeutics Provide Update on Results from MOVe-OUT Study of Molnupiravir, an Investigational Oral Antiviral Medicine, in At Risk Adults With Mild-to-Moderate COVID-19
Fischer, Eron, Holman, Cohen, Fang et al., Molnupiravir, an Oral Antiviral Treatment for COVID-19, doi:10.1101/2021.06.17.21258639
Kabinger, Stiller, Schmitzová, Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis, Nat Struct Mol Biol, doi:10.1038/s41594-021-00651-0
Kenilworth, Miami, Merck and Ridgeback to Present Phase 3 Data for Molnupiravir, an Investigational Oral COVID-19 Antiviral Medicine
Khoo, Fitzgerald, Fletcher, Ewings, Jaki et al., Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a Phase I, open-label, dose-escalating, randomized controlled study, J Antimicrob Chemother, doi:10.1093/jac/dkab318
Mahase, Covid-19: UK becomes first country to authorise antiviral molnupiravir, BMJ, doi:10.1136/bmj.n2697
Merck, Merck and Ridgeback Biotherapeutics provide update on progress of clinical development program for molnupiravir, an investigational oral therapeutic for the treatment of mild-to-moderate COVID-19
Painter, Holman, Bush, Almazedi, Malik et al., Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity Against SARS-CoV-2, Antimicrob Agents Chemother, doi:10.1128/AAC.02428-20
Painter, Sheahan, Baric, Holman, Donovan et al., Reduction in infectious SARS-CoV-2 in treatment study of COVID-19 with molnupiravir
Singh, Singh, Singh, Misra, Molnupiravir in COVID-19: A systematic review of literaturef, Diabetes Metab Syndr, doi:10.1016/j.dsx.2021.102329
DOI record:
{
"DOI": "10.2139/ssrn.4042673",
"ISSN": [
"1556-5068"
],
"URL": "http://dx.doi.org/10.2139/ssrn.4042673",
"author": [
{
"affiliation": [],
"family": "Tippabhotla",
"given": "Sudhakar Koudinya",
"sequence": "first"
},
{
"affiliation": [],
"family": "Lahiri",
"given": "Dr. Subhra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "D",
"given": "Rama Raju",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kandi",
"given": "Chandrashekhar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "V",
"given": "Naga Prasad",
"sequence": "additional"
}
],
"container-title": [
"SSRN Electronic Journal"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
3,
2
]
],
"date-time": "2022-03-02T03:07:25Z",
"timestamp": 1646190445000
},
"deposited": {
"date-parts": [
[
2022,
3,
2
]
],
"date-time": "2022-03-02T03:07:37Z",
"timestamp": 1646190457000
},
"indexed": {
"date-parts": [
[
2022,
3,
2
]
],
"date-time": "2022-03-02T16:12:54Z",
"timestamp": 1646237574430
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "1556-5068"
}
],
"issued": {
"date-parts": [
[
2022
]
]
},
"language": "en",
"member": "78",
"original-title": [],
"prefix": "10.2139",
"published": {
"date-parts": [
[
2022
]
]
},
"published-other": {
"date-parts": [
[
2022
]
]
},
"publisher": "Elsevier BV",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": null
}
},
"score": 1,
"short-container-title": [
"SSRN Journal"
],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"title": [
"Efficacy and Safety of Molnupiravir for the Treatment of Non-Hospitalized Adults With Mild COVID-19: A Randomized, Open-Label, Parallel-Group Phase 3 Trial"
],
"type": "journal-article"
}