Molnupiravir, an Oral Antiviral Treatment for COVID-19
et al., medRxiv, doi:10.1101/2021.06.17.21258639, NCT04405570, Jun 2021
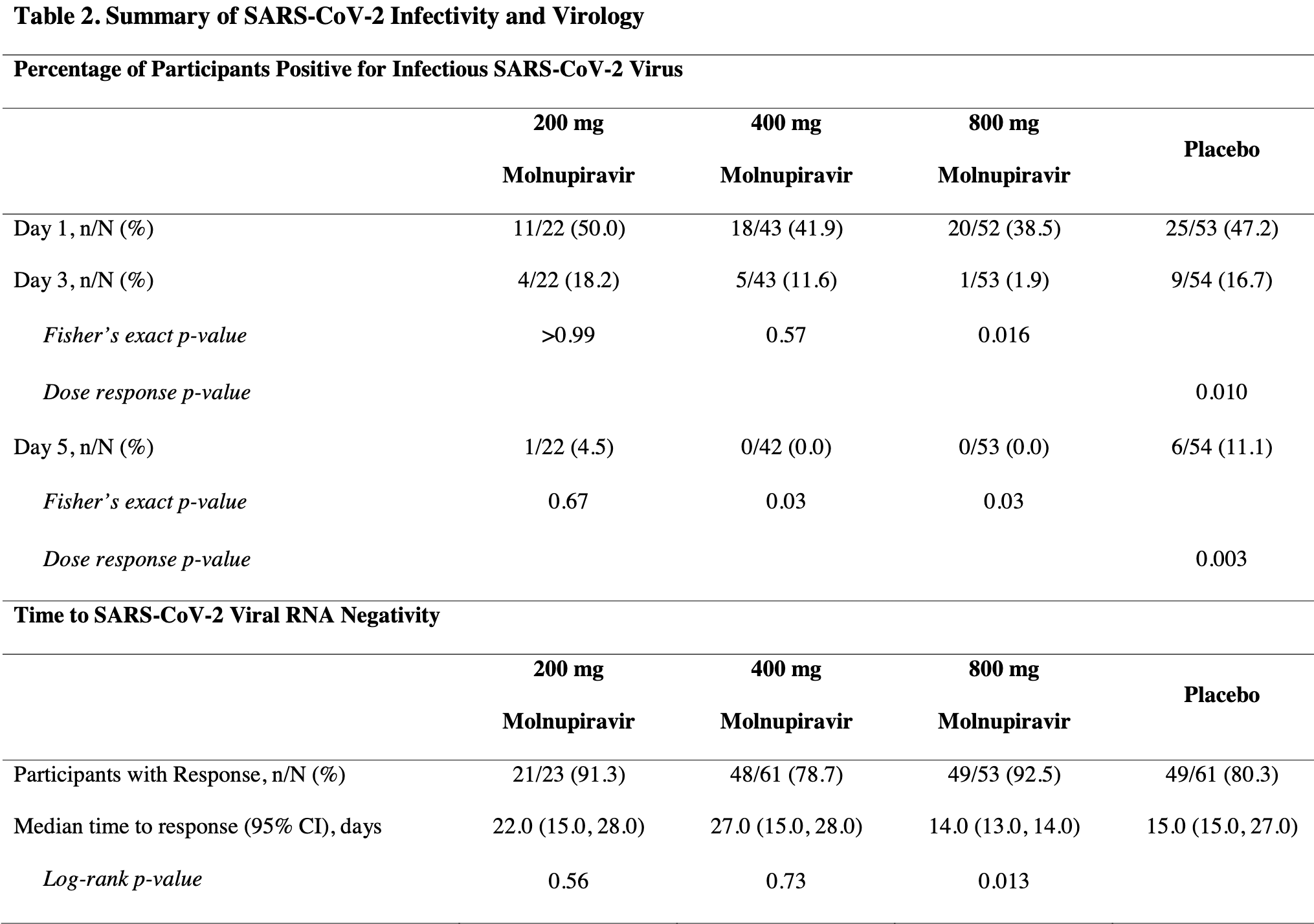
RCT 202 outpatients in the USA showing significantly faster viral clearance, but no significant differences in symptom duration or severity. NCT04405570 (history).
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments25.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 76.5% lower, RR 0.23, p = 0.31, treatment 0 of 140 (0.0%), control 1 of 62 (1.6%), NNT 62, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), all.
|
|
risk of death, 65.4% lower, RR 0.35, p = 1.00, treatment 0 of 55 (0.0%), control 1 of 62 (1.6%), NNT 62, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), 800mg.
|
|
risk of death, 66.7% lower, RR 0.33, p = 1.00, treatment 0 of 62 (0.0%), control 1 of 62 (1.6%), NNT 62, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), 400mg.
|
|
risk of death, 57.8% lower, RR 0.42, p = 1.00, treatment 0 of 23 (0.0%), control 1 of 62 (1.6%), NNT 62, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), 200mg.
|
|
risk of hospitalization, 32.9% higher, RR 1.33, p = 1.00, treatment 3 of 140 (2.1%), control 1 of 62 (1.6%), all.
|
|
risk of hospitalization, 12.7% higher, RR 1.13, p = 1.00, treatment 1 of 55 (1.8%), control 1 of 62 (1.6%), 800mg.
|
|
risk of hospitalization, 100% higher, RR 2.00, p = 1.00, treatment 2 of 62 (3.2%), control 1 of 62 (1.6%), 400mg.
|
|
risk of hospitalization, 57.8% lower, RR 0.42, p = 1.00, treatment 0 of 23 (0.0%), control 1 of 62 (1.6%), NNT 62, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), 200mg.
|
|
risk of no viral clearance, 49.2% lower, RR 0.51, p = 0.12, treatment 10 of 118 (8.5%), control 9 of 54 (16.7%), NNT 12, infectious, day 3, all.
|
|
risk of no viral clearance, 88.7% lower, RR 0.11, p = 0.02, treatment 1 of 53 (1.9%), control 9 of 54 (16.7%), NNT 6.8, infectious, day 3, 800mg.
|
|
risk of no viral clearance, 30.2% lower, RR 0.70, p = 0.57, treatment 5 of 43 (11.6%), control 9 of 54 (16.7%), NNT 20, infectious, day 3, 400mg.
|
|
risk of no viral clearance, 9.1% higher, RR 1.09, p = 1.00, treatment 4 of 22 (18.2%), control 9 of 54 (16.7%), infectious, day 3, 200mg.
|
|
risk of no viral clearance, 92.3% lower, RR 0.08, p = 0.004, treatment 1 of 117 (0.9%), control 6 of 54 (11.1%), NNT 9.8, infectious, day 5, all.
|
|
risk of no viral clearance, 92.2% lower, RR 0.08, p = 0.03, treatment 0 of 53 (0.0%), control 6 of 54 (11.1%), NNT 9.0, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), infectious, day 5, 800mg.
|
|
risk of no viral clearance, 91.4% lower, RR 0.09, p = 0.03, treatment 0 of 42 (0.0%), control 6 of 54 (11.1%), NNT 9.0, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), infectious, day 5, 400mg.
|
|
risk of no viral clearance, 59.1% lower, RR 0.41, p = 0.67, treatment 1 of 22 (4.5%), control 6 of 54 (11.1%), NNT 15, infectious, day 5, 200mg.
|
|
risk of no viral clearance, 29.5% lower, RR 0.70, p = 0.30, treatment 19 of 137 (13.9%), control 12 of 61 (19.7%), NNT 17, all.
|
|
risk of no viral clearance, 61.6% lower, RR 0.38, p = 0.10, treatment 4 of 53 (7.5%), control 12 of 61 (19.7%), NNT 8.2, 800mg.
|
|
risk of no viral clearance, 8.3% higher, RR 1.08, p = 1.00, treatment 13 of 61 (21.3%), control 12 of 61 (19.7%), 400mg.
|
|
risk of no viral clearance, 55.8% lower, RR 0.44, p = 0.33, treatment 2 of 23 (8.7%), control 12 of 61 (19.7%), NNT 9.1, 200mg.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
23.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Fischer et al., 18 Jun 2021, Randomized Controlled Trial, USA, preprint, 18 authors, study period 19 June, 2020 - 25 January, 2021, average treatment delay 4.6 days, trial NCT04405570 (history).
Molnupiravir, an Oral Antiviral Treatment for COVID-19
doi:10.1101/2021.06.17.21258639
Background: Easily distributed oral antivirals are urgently needed to treat coronavirus disease-2019 (COVID-19), prevent progression to severe illness, and block transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We report the results of a All rights reserved. No reuse allowed without permission. (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
Change from Baseline in SARS-CoV-2 Viral Load The decrease in viral RNA from baseline to Days 3 to 28 was greater for the 800 mg molnupiravir group than any other group (Table 2 and Figure 2c ). For participants administered 400 or 800 mg molnupiravir, the least squares mean viral load change from baseline was significantly greater on Day 5 when compared to placebo, with differences in least squares means of -0.434 and -0.547 log 10 copies/mL (p = 0.03 and 0.006), respectively. Additionally, for participants administered 800 mg molnupiravir, the least squares mean viral load change from baseline was also significantly greater on Day 7 compared to placebo, with a least squares mean difference of -0.534 log 10 copies/mL (p = 0.006). The reduction in viral load from baseline to Day 5 between 800 mg molnupiravir and placebo remained significant during sensitivity analyses of participants who were negative for antibodies at baseline (least squares mean difference of -0.613 log 10 copies/mL; p = 0.002; Supplementary Table 6 ) and when compared to concurrent placebo (least squares mean difference of -0.376 log 10 copies/mL; p = 0.045; Supplementary Table 8 ).
SARS-CoV-2 Antibody Detection Participants were tested for SARS-CoV-2-specific immunoglobulin (Ig) A, IgM, and IgG at baseline and on Days 7 and 28. The proportions of participants with any antibody to SARS-CoV-2 at baseline varied between the groups, with 15.0%, 30.0%, 35.3%, and 18.2% in the 200, 400, 800 mg..
References
Agostini, Pruijssers, Chappell, Small-molecule antiviral β-D-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance, J Virol
Aydillo, Gonzalez-Reiche, Aslam, Shedding of Viable SARS-CoV-2 after Immunosuppressive Therapy for Cancer, N Engl J Med
Bullard, Dust, Funk, Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From Diagnostic Samples, Clin Infect Dis Off Publ Infect Dis Soc Am
Chen, Nirula, Heller, SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19, N Engl J Med
Chen, Qi, Liu, Clinical progression of patients with COVID-19 in Shanghai, China, J Infect
Cox, Wolf, Plemper, Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets, Nat Microbiol
Folgueira, Luczkowiak, Lasala, Pérez-Rivilla, Delgado, Prolonged SARS-CoV-2 cell culture replication in respiratory samples from patients with severe COVID-19, Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis
González, Vielot, Sciaudone, Seroepidemiology of SARS-CoV-2 infections in an urban Nicaraguan population, MedRxiv Prepr Serv Health Sci
Gottlieb, Nirula, Chen, Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA
Liu, Yan, Wan, Viral dynamics in mild and severe cases of COVID-19, Lancet Infect Dis
Markmann, Giallourou, Bhowmik, Sex disparities and neutralizing antibody durability to SARS-CoV-2 infection in convalescent individuals, MedRxiv Prepr Serv Health Sci
Menachery, Yount, Debbink, A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence, Nat Med
Painter, Bowen, Bluemling, The prophylactic and therapeutic activity of a broadly active ribonucleoside analog in a murine model of intranasal venezuelan equine encephalitis virus infection, Antiviral Res
Painter, Holman, Bush, Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity Against SARS-CoV-2, Antimicrob Agents Chemother
Premkumar, Segovia-Chumbez, Jadi, The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients, Sci Immunol
Pruijssers, George, Schäfer, Remdesivir Inhibits SARS-CoV-2 in Human Lung Cells and Chimeric SARS-CoV Expressing the SARS-CoV-2 RNA Polymerase in Mice, Cell Rep
Sheahan, Sims, Zhou, An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 and multiple endemic, epidemic and bat coronavirus, Sci Transl Med
Singanayagam, Patel, Charlett, Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020, Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull
Van Kampen, Van De Vijver, Fraaij, Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19), Nat Commun
Wahl, Gralinski, Johnson, SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801, Nature
Weinreich, Sivapalasingam, Norton, REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19, N Engl J Med
Wölfel, Corman, Guggemos, Author Correction: Virological assessment of hospitalized patients with COVID-2019, Nature
DOI record:
{
"DOI": "10.1101/2021.06.17.21258639",
"URL": "http://dx.doi.org/10.1101/2021.06.17.21258639",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>Easily distributed oral antivirals are urgently needed to treat coronavirus disease-2019 (COVID-19), prevent progression to severe illness, and block transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We report the results of a Phase 2a trial evaluating the safety, tolerability, and antiviral efficacy of molnupiravir in the treatment of COVID-19 (<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"http://ClinicalTrials.gov\">ClinicalTrials.gov</jats:ext-link><jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04405570\">NCT04405570</jats:ext-link>).</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Eligible participants included outpatients with confirmed SARS-CoV-2 infection and symptom onset within 7 days. Participants were randomized 1:1 to 200 mg molnupiravir or placebo, or 3:1 to molnupiravir (400 or 800 mg) or placebo, twice-daily for 5 days. Antiviral activity was assessed as time to undetectable levels of viral RNA by reverse transcriptase polymerase chain reaction and time to elimination of infectious virus isolation from nasopharyngeal swabs.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Among 202 treated participants, virus isolation was significantly lower in participants receiving 800 mg molnupiravir (1.9%) versus placebo (16.7%) at Day 3 (p = 0.02). At Day 5, virus was not isolated from any participants receiving 400 or 800 mg molnupiravir, versus 11.1% of those receiving placebo (p = 0.03). Time to viral RNA clearance was decreased and a greater proportion overall achieved clearance in participants administered 800 mg molnupiravir versus placebo (p = 0.01). Molnupiravir was generally well tolerated, with similar numbers of adverse events across all groups.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Molnupiravir is the first oral, direct-acting antiviral shown to be highly effective at reducing nasopharyngeal SARS-CoV-2 infectious virus and viral RNA and has a favorable safety and tolerability profile.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2021,
6,
17
]
]
},
"author": [
{
"affiliation": [],
"family": "Fischer",
"given": "William",
"sequence": "first"
},
{
"affiliation": [],
"family": "Eron",
"given": "Joseph J.",
"sequence": "additional",
"suffix": "Jr"
},
{
"affiliation": [],
"family": "Holman",
"given": "Wayne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cohen",
"given": "Myron S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fang",
"given": "Lei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Szewczyk",
"given": "Laura J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sheahan",
"given": "Timothy P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baric",
"given": "Ralph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mollan",
"given": "Katie R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wolfe",
"given": "Cameron R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duke",
"given": "Elizabeth R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Azizad",
"given": "Masoud M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Borroto-Esoda",
"given": "Katyna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wohl",
"given": "David A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Loftis",
"given": "Amy James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alabanza",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lipansky",
"given": "Felicia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Painter",
"given": "Wendy P.",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
6,
18
]
],
"date-time": "2021-06-18T05:30:17Z",
"timestamp": 1623994217000
},
"deposited": {
"date-parts": [
[
2022,
12,
31
]
],
"date-time": "2022-12-31T15:29:53Z",
"timestamp": 1672500593000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2024,
3,
26
]
],
"date-time": "2024-03-26T23:10:51Z",
"timestamp": 1711494651280
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 122,
"issued": {
"date-parts": [
[
2021,
6,
17
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.06.17.21258639",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
6,
17
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
6,
17
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"key": "2021061908100663000_2021.06.17.21258639v1.1",
"unstructured": "World Health Organisation. Weekly epidemiological update on COVID-19 - 25 May 2021 [Internet]. Coronavirus Dis. COVID-19 Pandemic. [cited 2021 May 26];Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---25-may-2021"
},
{
"DOI": "10.1056/NEJMoa2029849",
"article-title": "SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19",
"doi-asserted-by": "crossref",
"first-page": "229",
"issue": "3",
"journal-title": "N Engl J Med",
"key": "2021061908100663000_2021.06.17.21258639v1.2",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2984-3",
"article-title": "Author Correction: Virological assessment of hospitalized patients with COVID-2019",
"doi-asserted-by": "crossref",
"first-page": "E35",
"issue": "7839",
"journal-title": "Nature",
"key": "2021061908100663000_2021.06.17.21258639v1.3",
"volume": "588",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-20568-4",
"doi-asserted-by": "publisher",
"key": "2021061908100663000_2021.06.17.21258639v1.4"
},
{
"DOI": "10.1016/S1473-3099(20)30232-2",
"article-title": "Viral dynamics in mild and severe cases of COVID-19",
"doi-asserted-by": "crossref",
"first-page": "656",
"issue": "6",
"journal-title": "Lancet Infect Dis",
"key": "2021061908100663000_2021.06.17.21258639v1.5",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.03.004",
"doi-asserted-by": "publisher",
"key": "2021061908100663000_2021.06.17.21258639v1.6"
},
{
"DOI": "10.1093/cid/ciaa638",
"article-title": "Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From Diagnostic Samples",
"doi-asserted-by": "crossref",
"first-page": "2663",
"issue": "10",
"journal-title": "Clin Infect Dis Off Publ Infect Dis Soc Am",
"key": "2021061908100663000_2021.06.17.21258639v1.7",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1038/s41564-020-00835-2",
"article-title": "Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets",
"doi-asserted-by": "crossref",
"first-page": "11",
"issue": "1",
"journal-title": "Nat Microbiol",
"key": "2021061908100663000_2021.06.17.21258639v1.8",
"volume": "6",
"year": "2021"
},
{
"article-title": "Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity Against SARS-CoV-2",
"first-page": "e02428",
"issue": "5",
"journal-title": "Antimicrob Agents Chemother",
"key": "2021061908100663000_2021.06.17.21258639v1.9",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2019.104597",
"doi-asserted-by": "publisher",
"key": "2021061908100663000_2021.06.17.21258639v1.10"
},
{
"article-title": "Small-molecule antiviral β-D-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance",
"first-page": "e01348",
"issue": "24",
"journal-title": "J Virol",
"key": "2021061908100663000_2021.06.17.21258639v1.11",
"volume": "93",
"year": "2019"
},
{
"DOI": "10.1126/scitranslmed.abb5883",
"doi-asserted-by": "publisher",
"key": "2021061908100663000_2021.06.17.21258639v1.12"
},
{
"DOI": "10.1016/j.celrep.2020.107940",
"doi-asserted-by": "publisher",
"key": "2021061908100663000_2021.06.17.21258639v1.13"
},
{
"DOI": "10.1038/s41586-021-03312-w",
"article-title": "SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801",
"doi-asserted-by": "crossref",
"first-page": "451",
"issue": "7850",
"journal-title": "Nature",
"key": "2021061908100663000_2021.06.17.21258639v1.14",
"volume": "591",
"year": "2021"
},
{
"DOI": "10.1038/nm.3985",
"doi-asserted-by": "publisher",
"key": "2021061908100663000_2021.06.17.21258639v1.15"
},
{
"DOI": "10.1056/NEJMc2031670",
"doi-asserted-by": "publisher",
"key": "2021061908100663000_2021.06.17.21258639v1.16"
},
{
"DOI": "10.1101/2021.02.01.21250493",
"doi-asserted-by": "publisher",
"key": "2021061908100663000_2021.06.17.21258639v1.17"
},
{
"DOI": "10.1126/sciimmunol.abc8413",
"doi-asserted-by": "crossref",
"key": "2021061908100663000_2021.06.17.21258639v1.18",
"unstructured": "Premkumar L , Segovia-Chumbez B , Jadi R , et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol 2020;5(48)."
},
{
"DOI": "10.1101/2021.02.25.21252447",
"doi-asserted-by": "publisher",
"key": "2021061908100663000_2021.06.17.21258639v1.19"
},
{
"article-title": "Prolonged SARS-CoV-2 cell culture replication in respiratory samples from patients with severe COVID-19",
"first-page": "886",
"issue": "6",
"journal-title": "Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis",
"key": "2021061908100663000_2021.06.17.21258639v1.20",
"volume": "27",
"year": "2021"
},
{
"article-title": "Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020",
"first-page": "2001483",
"issue": "32",
"journal-title": "Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull",
"key": "2021061908100663000_2021.06.17.21258639v1.21",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial",
"doi-asserted-by": "crossref",
"first-page": "632",
"issue": "7",
"journal-title": "JAMA",
"key": "2021061908100663000_2021.06.17.21258639v1.22",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19",
"doi-asserted-by": "crossref",
"first-page": "238",
"issue": "3",
"journal-title": "N Engl J Med",
"key": "2021061908100663000_2021.06.17.21258639v1.23",
"volume": "384",
"year": "2021"
},
{
"key": "2021061908100663000_2021.06.17.21258639v1.24",
"unstructured": "Regeneron Pharmaceuticals, Inc. Phase 3 Prevention Trial Showed 81% Reduced Risk of Symptomatic SARS-CoV-2 Infections with Subcutaneous Administration of REGEN-COV™(casirivimab with imdevimab) [Internet]. Cision PR Newswire. 2021 [cited 2021 May 12];Available from: https://www.prnewswire.com/news-releases/phase-3-prevention-trial-showed-81-reduced-risk-of-symptomatic-sars-cov-2-infections-with-subcutaneous-administration-of-regen-cov-casirivimab-with-imdevimab-301266366.html"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {
"is-preprint-of": [
{
"asserted-by": "subject",
"id": "10.1126/scitranslmed.abl7430",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2021.06.17.21258639"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Molnupiravir, an Oral Antiviral Treatment for COVID-19",
"type": "posted-content"
}