Paxlovid associated with decreased hospitalization rate among adults with COVID-19 — United States, April–September 2022
et al., American Journal of Transplantation, doi:10.1016/j.ajt.2022.12.004, Jan 2023
Retrospective 699,848 outpatients with COVID-19 showing lower hospitalization with paxlovid.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending paxlovid also recommended them, or
because the patient seeking out paxlovid is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Malden et al. confirm significant bias in the use of paxlovid, showing that treated
patients are more likely to be from affluent neighborhoods, be more health-conscious, and
have better access to care. Campion et al. also show that female patients were more
likely to receive paxlovid, and studies show that female patients are significantly more
likely to be health-conscious, for example being more likely to take additional
non-prescription treatments.
Therefore, these kind of studies may
overestimate efficacy.
Resistance. Variants may be resistant to paxlovid6-13. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID14. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid15. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid16. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury17 and liver injury18,19. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound20-22.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments23.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in the after exclusion results of meta-analysis:
only a fraction of eligible patients received treatment and these patients may be more likely to follow other recommendations, receive additional care, and more more likely to use additional untracked treatments such as vitamin D and nasal/oral hygiene.
|
risk of hospitalization, 51.0% lower, HR 0.49, p < 0.001, adjusted per study, multivariable, Cox proportional hazards, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Malden et al., Predictors of nirmatrelvir–ritonavir receipt among COVID-19 patients in a large US health system, Scientific Reports, doi:10.1038/s41598-024-57633-7.
5.
Campion et al., Disparities in the Use of nirmatrelvir/ritonavir for COVID-19: A Retrospective Cohort Study, Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1809.
6.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
7.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
8.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
9.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
10.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
11.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
12.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
13.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
14.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
15.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
16.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
17.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
18.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
19.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
20.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
21.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Shah et al., 31 Jan 2023, retrospective, USA, peer-reviewed, 11 authors.
Contact: bgn3@cdc.gov.
Report from the CDC: MMWR Paxlovid associated with decreased hospitalization rate among adults with COVID-19 -United States, April-September 2022
doi:10.1016/j.ajt.2022.12.
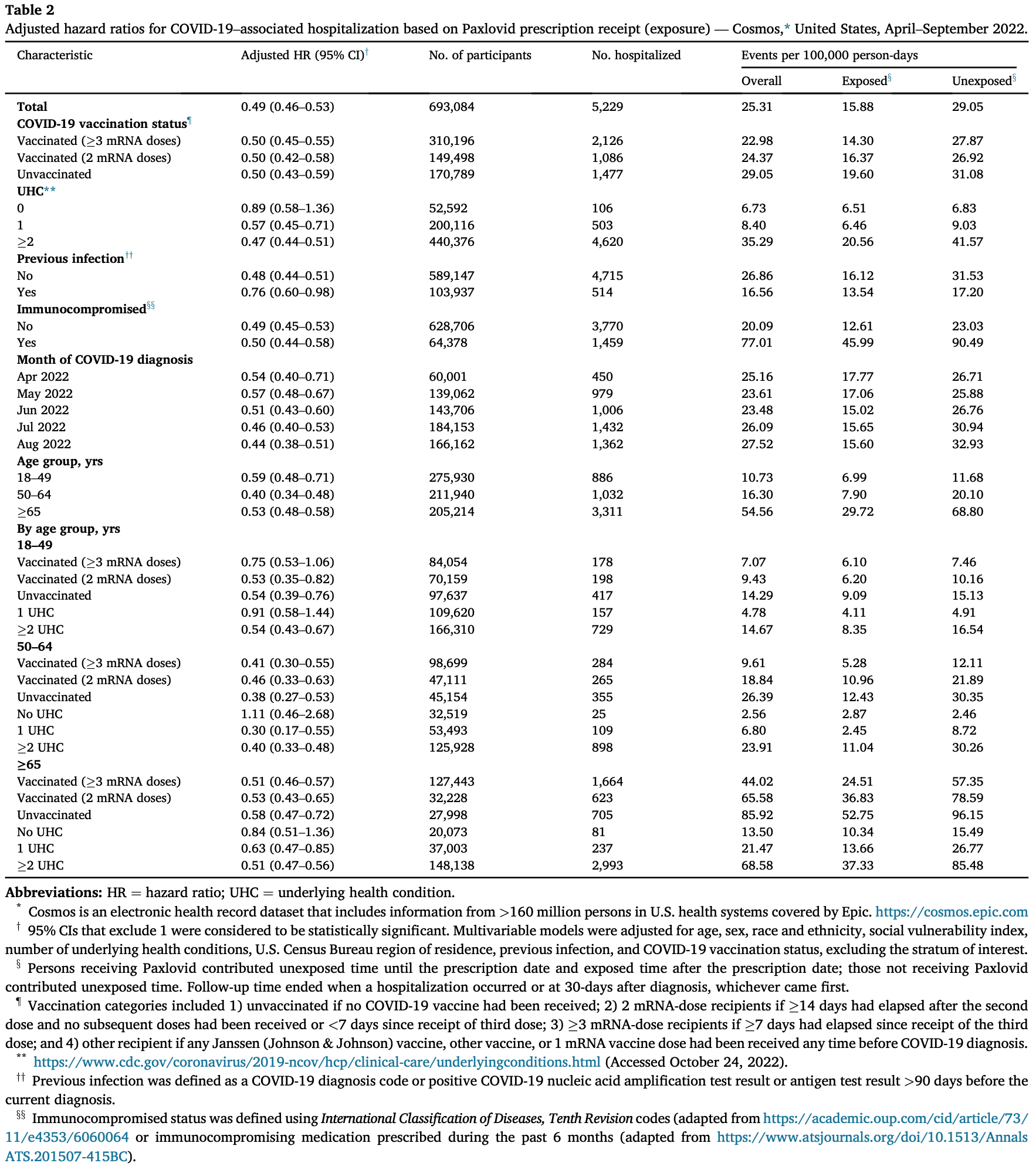
What is already known about this topic? Nirmatrelvir-ritonavir (Paxlovid) is an outpatient antiviral medication recommended for adults with mild-to-moderate COVID-19 who have elevated risk of severe illness. What is added by this report? Among U.S. adults diagnosed with COVID-19, including those with previous infection or vaccination, persons who were prescribed Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within 30 days after diagnosis than those who were not prescribed Paxlovid.
What are the implications for public health practice? Paxlovid should be offered to eligible adults irrespective of vaccination status, especially in groups with the highest risk for severe COVID-19 outcomes, such as older adults and those with multiple underlying health conditions. Nirmatrelvir-ritonavir (Paxlovid), an oral antiviral treatment, is authorized for adults with mild-to-moderate COVID-19 who are at increased risk for progression to severe illness. However, real-world evidence on the benefit of Paxlovid, according to vaccination status, age group, and underlying health conditions, is limited. To examine the benefit of Paxlovid in adults aged !18 years in the United States, a large electronic health record (EHR) data set (Cosmos y ) was analyzed to assess the association between receiving a prescription for Paxlovid and hospitalization with a COVID-19 diagnosis in the ensuing 30 days. A Cox proportional hazards model was used to estimate this association, adjusted for demographic characteristics, geographic location, vaccination, previous infection, and number of underlying health conditions. Among 699,848 adults aged !18 years eligible for Paxlovid during April-August 2022, 28.4% received a Paxlovid prescription within 5 days of COVID-19 diagnosis. Being prescribed Paxlovid was associated with a lower hospitalization rate among the overall study population (adjusted hazard ratio [aHR] ¼ 0.49), among those who had received !3 mRNA COVID-19 vaccines (aHR ¼ 0.50), and across age groups (18-49 years: aHR ¼ 0.59; 50-64 years: aHR ¼ 0.40; and !65 years: aHR ¼ 0.53). Paxlovid should be prescribed to eligible adults to reduce the risk of COVID-19-associated hospitalization. Paxlovid is an oral antiviral medication that received Emergency Use Authorization by the Food and Drug Administration on December 22, 2021 (1), for use in patients with mild-to-moderate COVID-19 at high risk for progression to severe illness. Eligibility for Paxlovid includes 1) receipt of a positive SARS-CoV-2 test result (including home antigen test), 2) symptoms consistent with mild-to-moderate COVID-19, 3) symptom onset within the past 5 days, 4) age !18 years (or age !12 years and weight !40 kg), 5) one or more risk factors for progression to severe COVID-19, 6) no known or suspected severe renal or hepatic impairment, 7) no history of clinically significant reactions (e.g., toxic epidermal necrolysis or Stevens-Johnson syndrome) to the active ingredients..
References
Arbel, Sagy, Hoshen, Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge, N Engl J Med, doi:10.1056/NEJMoa2204919
Dryden-Peterson, Kim, Kim, Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system, doi:10.1101/2022.06.14.22276393
Ganatra, Dani, Ahmad, Oral nirmatrelvir and ritonavir in nonhospitalized vaccinated patients with coronavirus disease 2019 (COVID-19), Clin Infect Dis, doi:10.1093/cid/ciac673
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Lewnard, Malden, Hong, Effectiveness of nirmatrelvir-ritonavir against hospital admission: a matched cohort study in a large US healthcare system, doi:10.1101/2022.10.02.22280623
Najjar-Debbiny, Gronich, Weber, Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients, Clin Infect Dis, doi:10.1093/cid/ciac443
Vangeel, Chiu, Jonghe, Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern, Antiviral Res, doi:10.1016/j.antiviral.2022.105252
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir among COVID-19 inpatients during Hong Kong's Omicron BA.2 wave: an observational study, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00507-2
Yek, Warner, Wiltz, Risk factors for severe COVID-19 outcomes among persons aged !18 years who completed a primary COVID-19 vaccination series-465 health care facilities, United States, December 2020-October 2021, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7101a4
DOI record:
{
"DOI": "10.1016/j.ajt.2022.12.004",
"ISSN": [
"1600-6135"
],
"URL": "http://dx.doi.org/10.1016/j.ajt.2022.12.004",
"alternative-id": [
"S160061352229279X"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Paxlovid associated with decreased hospitalization rate among adults with COVID-19 — United States, April–September 2022"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "American Journal of Transplantation"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ajt.2022.12.004"
},
{
"label": "Content Type",
"name": "content_type",
"value": "simple-article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 Published by Elsevier Inc. on behalf of American Society of Transplantation & American Society of Transplant Surgeons."
}
],
"author": [
{
"affiliation": [],
"family": "Shah",
"given": "Melisa M.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Joyce",
"given": "Brendan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Plumb",
"given": "Ian D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sahakian",
"given": "Sam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Feldstein",
"given": "Leora R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barkley",
"given": "Eric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paccione",
"given": "Mason",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deckert",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sandmann",
"given": "Danessa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gerhart",
"given": "Jacqueline L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hagen",
"given": "Melissa Briggs",
"sequence": "additional"
}
],
"container-title": "American Journal of Transplantation",
"container-title-short": "American Journal of Transplantation",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"amjtransplant.org",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
1,
11
]
],
"date-time": "2023-01-11T23:01:26Z",
"timestamp": 1673478086000
},
"deposited": {
"date-parts": [
[
2024,
1,
1
]
],
"date-time": "2024-01-01T02:52:04Z",
"timestamp": 1704077524000
},
"funder": [
{
"DOI": "10.13039/100000030",
"doi-asserted-by": "publisher",
"name": "Centers for Disease Control and Prevention"
}
],
"indexed": {
"date-parts": [
[
2024,
2,
29
]
],
"date-time": "2024-02-29T12:01:29Z",
"timestamp": 1709208089784
},
"is-referenced-by-count": 15,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2023,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
1
]
],
"date-time": "2023-01-01T00:00:00Z",
"timestamp": 1672531200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 365,
"start": {
"date-parts": [
[
2024,
1,
1
]
],
"date-time": "2024-01-01T00:00:00Z",
"timestamp": 1704067200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S160061352229279X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S160061352229279X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "150-155",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
1
]
]
},
"published-print": {
"date-parts": [
[
2023,
1
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.ajt.2022.12.004_bib1",
"series-title": "Paxlovid emergency use authorization. Silver Spring",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ajt.2022.12.004_bib2",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2204919",
"article-title": "Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge",
"author": "Arbel",
"doi-asserted-by": "crossref",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ajt.2022.12.004_bib3",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1101/2022.06.14.22276393",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ajt.2022.12.004_bib4",
"unstructured": "Dryden-Peterson S, Kim A, Kim AY, et al. Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system. medRxiv [Preprint posted June 17, 2022]. https://doi.org/10.1101/2022.06.14.22276393"
},
{
"DOI": "10.1101/2022.10.02.22280623",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ajt.2022.12.004_bib5",
"unstructured": "Lewnard JA, Malden D, Hong V, et al. Effectiveness of nirmatrelvir-ritonavir against hospital admission: a matched cohort study in a large US healthcare system. medRxiv [Preprint posted October 4, 2022]. https://doi.org/10.1101/2022.10.02.22280623"
},
{
"article-title": "Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir among COVID-19 inpatients during Hong Kong’s Omicron BA.2 wave: an observational study",
"author": "Wong",
"issue": "22",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.ajt.2022.12.004_bib6",
"volume": "S14373–3099",
"year": "2022"
},
{
"article-title": "Oral nirmatrelvir and ritonavir in nonhospitalized vaccinated patients with coronavirus disease 2019 (COVID-19)",
"author": "Ganatra",
"first-page": "ciac673",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ajt.2022.12.004_bib7",
"year": "2022"
},
{
"article-title": "Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients",
"author": "Najjar-Debbiny",
"first-page": "ciac443",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ajt.2022.12.004_bib8",
"year": "2022"
},
{
"article-title": "Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern",
"author": "Vangeel",
"first-page": "198",
"journal-title": "Antiviral Res",
"key": "10.1016/j.ajt.2022.12.004_bib9",
"volume": "105252",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7101a4",
"article-title": "Risk factors for severe COVID-19 outcomes among persons aged ≥18 years who completed a primary COVID-19 vaccination series—465 health care facilities, United States, December 2020–October 2021",
"author": "Yek",
"doi-asserted-by": "crossref",
"first-page": "19",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "10.1016/j.ajt.2022.12.004_bib10",
"volume": "71",
"year": "2022"
}
],
"reference-count": 10,
"references-count": 10,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S160061352229279X"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Transplantation",
"Immunology and Allergy"
],
"subtitle": [],
"title": "Paxlovid associated with decreased hospitalization rate among adults with COVID-19 — United States, April–September 2022",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "23"
}