Effect of a Nutritional Support System to Increase Survival and Reduce Mortality in Patients with COVID-19 in Stage III and Comorbidities: A Blinded Randomized Controlled Clinical Trial
et al., International Journal of Environmental Research and Public Health, doi:10.3390/ijerph19031172, NCT04507867, Oct 2021 (preprint)
Zinc for COVID-19
2nd treatment shown to reduce risk in
July 2020, now with p = 0.00000019 from 42 studies, recognized in 23 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
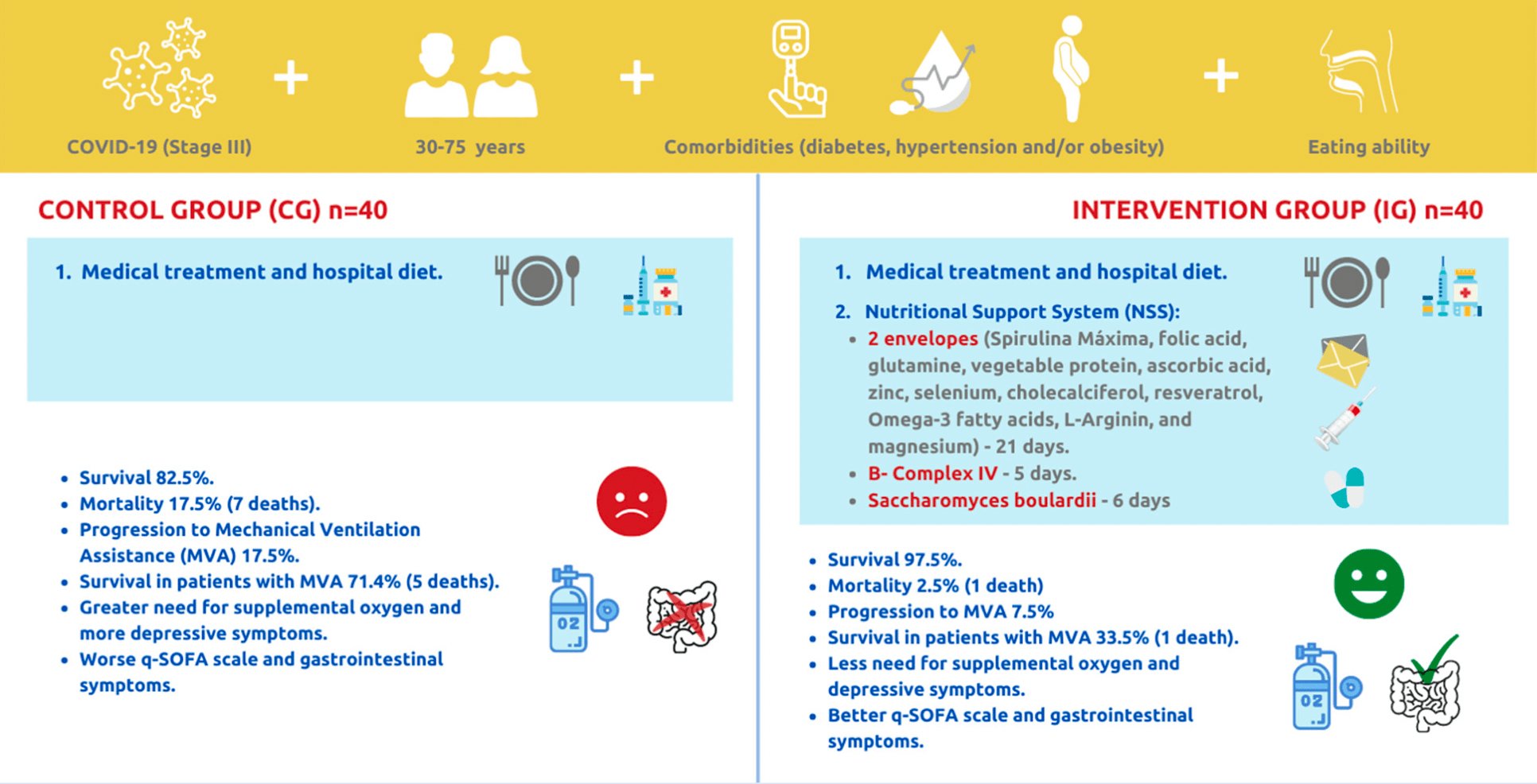
80 patient RCT with 40 patients treated with a comprehensive regimen of nutritional support, showing significantly lower mortality with treatment. Treatment contained cholecalciferol, vitamin C, zinc, spirulina maxima, folic acid, glutamine, vegetable protein, selenium, resveratrol, omega-3 fatty acids, l-arginine, magnesium, probiotics, and B-complex IV. Adherence was strictly monitored.
This study is excluded in the after exclusion results of meta-analysis:
combined treatments may contribute more to the effect seen.
|
risk of death, 85.7% lower, RR 0.14, p = 0.03, treatment 1 of 40 (2.5%), control 7 of 40 (17.5%), NNT 6.7.
|
|
risk of mechanical ventilation, 57.1% lower, RR 0.43, p = 0.31, treatment 3 of 40 (7.5%), control 7 of 40 (17.5%), NNT 10.0.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Leal-Martínez et al., 25 Oct 2021, Randomized Controlled Trial, Mexico, peer-reviewed, 7 authors, study period 1 September, 2020 - 28 February, 2021, this trial uses multiple treatments in the treatment arm (combined with comprehensive nutritional support) - results of individual treatments may vary, trial NCT04507867 (history).
Effect of a Nutritional Support System to Increase Survival and Reduce Mortality in Patients with COVID-19 in Stage III and Comorbidities: A Blinded Randomized Controlled Clinical Trial
International Journal of Environmental Research and Public Health, doi:10.3390/ijerph19031172
The COVID-19 evolution depends on immunological capacity. The global hospital mortality rate is 15-20%, but in México it is 46%. There are several therapeutic protocols, however, integral nutrition is not considered. In this study, a Nutritional Support System (NSS) was employed to increase survival and reduce mortality in patients with stage III COVID-19. A randomized, blinded, controlled clinical trial was performed. Eighty patients (aged 30 to 75 years, both sexes) were assigned to (1) "Control Group" (CG) hospital diet and medical treatment or (2) "Intervention Group" (IG) hospital diet, medical treatment, and the NSS (vitamins, minerals, fiber, omega-3, amino acids, Bcomplex, and probiotics). IG significantly increased survival and reduced mortality compared to CG (p = 0.027). IG decreased progression to Mechanical Ventilation Assistance (MVA) by 10%, reduced the intubation period by 15 days, and increased survival in intubated patients by 38% compared to CG. IG showed improvement compared to CG in decrease in supplemental oxygen (p = 0.014), the qSOFA test (p = 0.040), constipation (p = 0.014), the PHQ-9 test (p = 0.003), and in the follow-up, saturation with oxygen (p = 0.030). The NSS increases survival and decreases mortality in patients with stage III COVID-19.
Conflicts of Interest: The authors declare that they have no conflict of interest.
References
Abdrabbo, Birch, Brandt, Cicigoi, Coffey et al., Vitamin D and COVID-19: A review on the role of vitamin D in preventing and reducing the severity of COVID-19 infection, Protein Sci, doi:10.1002/pro.4190
Acosta-Elias, Espinosa-Tanguma, The Folate Concentration and/or Folic Acid Metabolites in Plasma as Factor for COVID-19 Infection, Front. Pharmacol, doi:10.3389/fphar.2020.01062
Adebayo, Varzideh, Wilson, Gambardella, Eacobacci et al., l-Arginine y COVID-19: An Update, Nutrients, doi:10.3390/nu13113951
Al Sulaiman, Aljuhani, Al Dossari, Alshahrani, Alharbi et al., Evaluation of thiamine as adjunctive therapy in COVID-19 critically ill patients: A two-center propensity score matched study, Crit. Care, doi:10.1186/s13054-021-03648-9
Anand, Mande, Diet, Microbiota and Gut-Lung Connection, Front. Microbiol, doi:10.3389/fmicb.2018.02147
Anker, Landmesser, Von Haehling, Butler, Coats et al., Weight loss, malnutrition, and cachexia in COVID-19: Facts and numbers, J. Cachexia Sarcopenia Muscle, doi:10.1002/jcsm.12674
Barazzoni, Bischoff, Breda, Wickramasinghe, Krznaric et al., endorsed by the ESPEN Council. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection, Clin. Nutr, doi:10.1016/j.clnu.2020.03.022
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the Treatment of COVID-19-preliminary report, N. Engl. J. Med, doi:10.1056/NEJMoa2007764
Bermano, Méplan, Mercer, Hesketh, Selenium and viral infection: Are there lessons for COVID-19?, Br. J. Nutr, doi:10.1017/S0007114520003128
Bharadwaj, Singh, Kirtipal, Kang, SARS-CoV-2 and Glutamine: SARS-CoV-2 Triggered Pathogenesis via Metabolic Reprograming of Glutamine in Host Cells, Front. Mol. Biosci, doi:10.3389/fmolb.2020.627842
Bhimraj, Morgan, Shumaker, Lavergne, Baden et al., Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with (COVID-19), IDSA, doi:10.1093/cid/ciaa478
Birkeland, Gharagozlian, Birkeland, Valeur, Måge et al., Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: A randomized controlled trial, Eur. J. Nutr, doi:10.1007/s00394-020-02282-5
Cardinali, Brown, Pandi-Perumal, Can Melatonin Be a Potential "Silver Bullet" in Treating COVID-19 Patients, Diseases, doi:10.3390/diseases8040044
Castro, Rodríguez, Levaduras: Probióticos y prebióticos para mejorar la producción animal, Ciencia y Tecnología Agropecuaria, doi:10.21930/rcta.vol6_num1_art:33
Cengiz, Uysal, Ikitimur, Ozcan, Islamo Glu et al., Effect of oral l-Glutamine supplementation on Covid-19 treatment, Clin. Nutr. Exp, doi:10.1016/j.yclnex.2020.07.003
Chen, Zhou, Dong, Qu, Gong et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study, Lancet
Covid19, Who, WHO Coronavirus (COVID-19) Dashboard
Djokic, Vojvodic, Korcok, Agic, Rankovic et al., The Effects of Magnesium-Melatonin-Vit B Complex Supplementation in Treatment of Insomnia, Open Access Maced. J. Med. Sci, doi:10.3889/oamjms.2019.771
Doboszewska, Wlaź, Nowak, Młyniec, Targeting zinc metalloenzymes in COVID-19, Br. J. Pharmacol, doi:10.1111/bph.15199
Dy, Tanyaratsrisakul, Voelker, Ledford, The Emerging Roles of Surfactant Protein-A in Asthma, J. Clin. Cell. Immunol, doi:10.4172/2155-9899.1000553
Fowler, Iii, None
Gheorghe, Martin, Manriquez, Dinan, Cryan et al., Focus on the essentials: Tryptophan metabolism and the microbiome-gut-brain axis, Curr. Opin. Pharmacol, doi:10.1016/j.coph.2019.08.004
Hamanaka, O'leary, Witt, Tian, Gökalp et al., Glutamine Metabolism Is Required for Collagen Protein Synthesis in Lung Fibroblasts, Am. J. Respir. Cell Mol. Biol, doi:10.1165/rcmb.2019-0008OC
Hao, Dong, Wu, Probiotics for preventing acute upper respiratory tract infections, Cochrane Database Syst. Rev, doi:10.1002/14651858.CD006895.pub3
Iddir, Brito, Dingeo, Del Campo, Samouda et al., Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis, Nutrients, doi:10.3390/nu12061562
Infusino, Marazzato, Mancone, Fedele, Mastroianni et al., Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review, Nutrients, doi:10.3390/nu12061718
Kaźmierczak-Siedlecka, Ruszkowski, Fic, Folwarski, Makarewicz, Saccharomyces boulardii CNCM I-745: A Non-bacterial Microorganism Used as Probiotic Agent in Supporting Treatment of Selected Diseases, Curr. Microbiol, doi:10.1007/s00284-020-02053-9
Kory, Meduri, Iglesias, Varon, Marik, Clinical and Scientific Rationale for the "MATH+" Hospital Treatment Protocol for COVID-19, J. Intensiv. Care Med, doi:10.1177/0885066620973585
Leguía Valentín, Niño Montero, Quino Florentini, Coronavirus causante del síndrome respiratorio de Oriente Medio (MERS-CoV), Rev. Med. Carrion
Ling, Broad, Murphy, Pappachan, Pardesi-Newton et al., High-Dose Cholecalciferol Booster Therapy is Associated with a Reduced Risk of Mortality in Patients with COVID-19: A Cross-Sectional Multi-Centre Observational Study, Nutrients, doi:10.3390/nu12123799
Louca, Murray, Klaser, Graham, Mazidi et al., Modest effects of dietary supplements during the COVID-19 pandemic: Insights from 445 850 users of the COVID-19 Symptom Study app, BMJ Nutr. Prev. Health, doi:10.1136/bmjnph-2021-000250
Malik, Suboc, Tyagi, Salzman, Wang et al., Lactobacillus plantarum 299v Supplementation Improves Vascular Endothelial Function and Reduces Inflammatory Biomarkers in Men with Stable Coronary Artery Disease, Circ. Res, doi:10.1161/CIRCRESAHA.118.313565
Maneira, Bermejo, Pereira, De Mello, Exploring G protein-coupled receptors and yeast surface display strategies for viral detection in baker's yeast: SARS-CoV-2 as a case study, FEMS Yeast Res, doi:10.1093/femsyr/foab004
Mejia, Alvarado, Vitamina C como antioxidante en el manejo del SARS-CoV-2, Rev. Colomb. Endocrinol. Diabetes Metab, doi:10.53853/encr.7.2S.593
Munshi, Hussein, Toraih, Elshazli, Jardak et al., Vitamin D insufficiency as a potential culprit in critical COVID-19 patients, J. Med. Virol, doi:10.1002/jmv.26360
Narayanan, Nair, Vitamin B12 may inhibit RNA-dependent-RNA polymerase activity of nsp12 from the SARS-CoV-2 virus, IUBMB Life, doi:10.1002/iub.2359
Natarajan, Brophy, Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients with Sepsis and Severe Acute Respiratory Failure, JAMA, doi:10.1001/jama.2019.11825
Pormohammad, Monych, Turner, Zinc and SARS-CoV-2: A molecular modeling study of Zn interactions with RNA-dependent RNA-polymerase and 3C-like proteinase enzymes, Int. J. Mol. Med, doi:10.3892/ijmm.2020.4790
Pujadas, Chaudhry, Mcbride, Richter, Zhao et al., SARS-CoV-2 viral load predicts COVID-19 mortality, Lancet Respir. Med, doi:10.1016/S2213-2600(20)30354-4
Rakib, Nain, Sami, Mahmud, Islam et al., A molecular modelling approach for identifying antiviral selenium-containing heterocyclic compounds that inhibit the main protease of SARS-CoV-2: An in-silico investigation, Brief. Bioinform, doi:10.1093/bib/bbab045
Ramdani, Bachari, Potential therapeutic effects of Resveratrol against SARS-CoV-2, Acta Virol, doi:10.4149/av_2020_309
Ratha, Renuka, Rawat, Bux, Prospective options of algae-derived nutraceuticals as supplements to combat COVID-19 and human coronavirus diseases, Nutrition, doi:10.1016/j.nut.2020.111089
Reizine, Lesouhaitier, Gregoire, Pinceaux, Gacouin et al., SARS-CoV-2-Induced ARDS Associates with MDSC Expansion, Lymphocyte Dysfunction, and Arginine Shortage, J. Clin. Immunol, doi:10.1007/s10875-020-00920-5
Rogero, Leão, Santana, Pimentel, Carlini et al., Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19, Free Radic. Biol. Med, doi:10.1016/j.freeradbiomed.2020.07.005
Salama, Han, Yau, Reiss, Kramer et al., Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia, N. Engl. J. Med, doi:10.1056/NEJMoa2030340
Salud, Coronavirus COVID19 Comunicado Técnico Diario
Salud, Edomex, Estadísticas COVID-19|Centro Estatal de Vigilancia Epidemiológica y Control de Enfermedades
Serseg, Benarous, Yousfi, Hispidin, Lepidine, Two Natural Compounds and Folic Acid as Potential Inhibitors of 2019-novel Coronavirus Main Protease (2019-nCoVMpro), Molecular Docking and SAR Study, Curr. Comput. Aided Drug Des, doi:10.2174/1573409916666200422075440
Shakoor, Feehan, Mikkelsen, Al Dhaheri, Ali et al., Be well: A potential role for vitamin B in COVID-19, Maturitas, doi:10.1016/j.maturitas.2020.08.007
Sidawi, Garau, COVID-19 Mortality. Trends in the evolution of the pandemic, J. Health Sci, doi:10.3306/MEDICINABALEAR.36.01.42
Sousa Cavalcante, Costa-Silva, Souza, Ienne, Monteiro, Chemogenomic study of gemcitabine using Saccharomyces cerevisiae as model cell-Molecular insights about chemoresistance, Braz. J. Microbiol, doi:10.1007/s42770-019-00154-7
Staples, Wall, Li, Tipple, Selenium-independent antioxidant and anti-inflammatory effects of thioredoxin reductase inhibition in alveolar macrophages, Life Sci, doi:10.1016/j.lfs.2020.118285
Talanquer, La Letalidad Hospitalaria por COVID-19 en México: Desigualdades Institucionales
Tian, Hu, Lou, Chen, Kang et al., Characteristics of COVID-19 infection in Beijing, J. Infect, doi:10.1016/j.jinf.2020.02.018
Truwit, Hite, Morris, Dewilde, Priday et al., None
Van Der Beek, Canfora, Kip, Gorissen, Olde Damink et al., The prebiotic inulin improves substrate metabolism and promotes short-chain fatty acid production in overweight to obese men, Metabolism, doi:10.1016/j.metabol.2018.06.009
Van Kempen, Deixler, SARS-CoV-2: Influence of phosphate and magnesium, moderated by vitamin D, on energy (ATP) metabolism and on severity of COVID-19, Am. J. Physiol. Endocrinol. Metab, doi:10.1152/ajpendo.00474.2020
Vanhove, Derveaux, Graulus, Mesotten, Thomeer et al., Glutamine Addiction and Therapeutic Strategies in Lung Cancer, Int. J. Mol. Sci, doi:10.3390/ijms20020252
Virgens, Santana, Lima, Fayh, Can COVID-19 be a risk for cachexia for patients during intensive care? Narrative review and nutritional recommendations, Br. J. Nutr, doi:10.1017/S0007114520004420
Vollbracht, Kraft, Feasibility of Vitamin C in the Treatment of Post Viral Fatigue with Focus on Long COVID, Based on a Systematic Review of IV Vitamin C on Fatigue, Nutrients, doi:10.3390/nu13041154
Wang, Liu, Liu, Wang, Luo et al., Clinical Outcomes in 55 Patients with Severe Acute Respiratory Syndrome Coronavirus 2 Who Were Asymptomatic at Hospital Admission in Shenzhen, China, J. Infect. Dis, doi:10.1093/infdis/jiaa119
Weill, Plissonneau, Legrand, Rioux, Thibault, May omega-3 fatty acid dietary supplementation help reduce severe complications in Covid-19 patients?, Biochimie, doi:10.1016/j.biochi.2020.09.003
Zhang, Guo, Lei, Liu, Wang et al., Frontline Science: COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes, J. Leukoc. Biol, doi:10.1002/JLB.4HI0720-470R
Zhao, Li, Ge, Shi, Lv et al., Evaluation of Nutrition Risk and Its Association With Mortality Risk in Severely and Critically Ill COVID-19 Patients, JPEN J. Parenter. Enter. Nutr
Zhu, Cai, Fan, Lou, Hua et al., Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.04.041
Zuo, Zhang, Lui, Yeoh, Li et al., Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization, Gastroenterology, doi:10.1053/j.gastro.2020.05.048
DOI record:
{
"DOI": "10.3390/ijerph19031172",
"ISSN": [
"1660-4601"
],
"URL": "http://dx.doi.org/10.3390/ijerph19031172",
"abstract": "<jats:p>The COVID-19 evolution depends on immunological capacity. The global hospital mortality rate is 15–20%, but in México it is 46%. There are several therapeutic protocols, however, integral nutrition is not considered. In this study, a Nutritional Support System (NSS) was employed to increase survival and reduce mortality in patients with stage III COVID-19. A randomized, blinded, controlled clinical trial was performed. Eighty patients (aged 30 to 75 years, both sexes) were assigned to (1) “Control Group” (CG) hospital diet and medical treatment or (2) “Intervention Group” (IG) hospital diet, medical treatment, and the NSS (vitamins, minerals, fiber, omega-3, amino acids, B-complex, and probiotics). IG significantly increased survival and reduced mortality compared to CG (p = 0.027). IG decreased progression to Mechanical Ventilation Assistance (MVA) by 10%, reduced the intubation period by 15 days, and increased survival in intubated patients by 38% compared to CG. IG showed improvement compared to CG in decrease in supplemental oxygen (p = 0.014), the qSOFA test (p = 0.040), constipation (p = 0.014), the PHQ-9 test (p = 0.003), and in the follow-up, saturation with oxygen (p = 0.030). The NSS increases survival and decreases mortality in patients with stage III COVID-19.</jats:p>",
"alternative-id": [
"ijerph19031172"
],
"author": [
{
"affiliation": [],
"family": "Leal-Martínez",
"given": "Fernando",
"sequence": "first"
},
{
"affiliation": [],
"family": "Abarca-Bernal",
"given": "Lorena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García-Pérez",
"given": "Alejandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "González-Tolosa",
"given": "Dinnaru",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cruz-Cázares",
"given": "Georgina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montell-García",
"given": "Marco",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2489-4689",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ibarra",
"given": "Antonio",
"sequence": "additional"
}
],
"container-title": [
"International Journal of Environmental Research and Public Health"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
21
]
],
"date-time": "2022-01-21T13:37:18Z",
"timestamp": 1642772238000
},
"deposited": {
"date-parts": [
[
2022,
1,
21
]
],
"date-time": "2022-01-21T14:23:36Z",
"timestamp": 1642775016000
},
"indexed": {
"date-parts": [
[
2022,
1,
21
]
],
"date-time": "2022-01-21T14:43:20Z",
"timestamp": 1642776200615
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "1660-4601"
}
],
"issue": "3",
"issued": {
"date-parts": [
[
2022,
1,
21
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2022,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
21
]
],
"date-time": "2022-01-21T00:00:00Z",
"timestamp": 1642723200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1660-4601/19/3/1172/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1172",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
1,
21
]
]
},
"published-online": {
"date-parts": [
[
2022,
1,
21
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1016/j.jinf.2020.02.018",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"article-title": "Coronavirus causante del síndrome respiratorio de Oriente Medio (MERS-CoV)",
"author": "Leguía Valentín",
"first-page": "1",
"journal-title": "Rev. Med. Carrion.",
"key": "ref2",
"volume": "1",
"year": "2019"
},
{
"key": "ref3",
"unstructured": "WHO Coronavirus (COVID-19) Dashboard\nhttps://covid19.who.int/"
},
{
"key": "ref4",
"unstructured": "https://www.gob.mx/salud/documentos/coronavirus-covid-19-comunicado-tecnico-diario-238449"
},
{
"key": "ref5",
"unstructured": "https://datos.covid-19.conacyt.mx/"
},
{
"key": "ref6",
"unstructured": "https://salud.edomex.gob.mx/cevece/estadisticas_covid19"
},
{
"key": "ref7",
"unstructured": "https://datos.nexos.com.mx/la-letalidad-hospitalaria-por-covid-19-en-mexico-desigualdades-institucionales/"
},
{
"DOI": "10.1093/infdis/jiaa119",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1093/cid/ciaa478",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1016/j.clnu.2020.03.022",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1136/bmjnph-2021-000250",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.21930/rcta.vol6_num1_art:33",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.3389/fmicb.2018.02147",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1016/S2213-2600(20)30354-4",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1186/s13054-021-03648-9",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1016/j.maturitas.2020.08.007",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1016/j.nut.2020.111089",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.3389/fphar.2020.01062",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.2174/1573409916666200422075440",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1016/j.yclnex.2020.07.003",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.3389/fmolb.2020.627842",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.3390/ijms20020252",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.4172/2155-9899.1000553",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1165/rcmb.2019-0008OC",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.3390/nu12061562",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1093/femsyr/foab004",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1007/s42770-019-00154-7",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1001/jama.2019.11825",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.53853/encr.7.2S.593",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.3390/nu13041154",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.3892/ijmm.2020.4790",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1111/bph.15199",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1017/S0007114520003128",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1093/bib/bbab045",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1016/j.lfs.2020.118285",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.1161/CIRCRESAHA.118.313565",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.3390/nu12123799",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1002/pro.4190",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.4149/av_2020_309",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1016/j.biochi.2020.09.003",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1007/s10875-020-00920-5",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1152/ajpendo.00474.2020",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1007/s00284-020-02053-9",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1056/NEJMoa2030340",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1002/jpen.1953",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.3390/nu13113951",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.1177/0885066620973585",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.1002/jcsm.12674",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.3889/oamjms.2019.771",
"doi-asserted-by": "publisher",
"key": "ref51"
},
{
"DOI": "10.3306/MEDICINABALEAR.36.01.42",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.1016/j.ijid.2020.04.041",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"DOI": "10.1053/j.gastro.2020.05.048",
"doi-asserted-by": "publisher",
"key": "ref54"
},
{
"DOI": "10.1002/14651858.CD006895.pub3",
"doi-asserted-by": "publisher",
"key": "ref55"
},
{
"DOI": "10.3390/nu12061718",
"doi-asserted-by": "publisher",
"key": "ref56"
},
{
"DOI": "10.1002/jmv.26360",
"doi-asserted-by": "publisher",
"key": "ref57"
},
{
"DOI": "10.1002/JLB.4HI0720-470R",
"doi-asserted-by": "publisher",
"key": "ref58"
},
{
"DOI": "10.1016/j.freeradbiomed.2020.07.005",
"doi-asserted-by": "publisher",
"key": "ref59"
},
{
"DOI": "10.1016/j.metabol.2018.06.009",
"doi-asserted-by": "publisher",
"key": "ref60"
},
{
"DOI": "10.1007/s00394-020-02282-5",
"doi-asserted-by": "publisher",
"key": "ref61"
},
{
"DOI": "10.1002/iub.2359",
"doi-asserted-by": "publisher",
"key": "ref62"
},
{
"DOI": "10.1017/S0007114520004420",
"doi-asserted-by": "publisher",
"key": "ref63"
},
{
"DOI": "10.3390/diseases8040044",
"doi-asserted-by": "publisher",
"key": "ref64"
},
{
"DOI": "10.1016/j.coph.2019.08.004",
"doi-asserted-by": "publisher",
"key": "ref65"
}
],
"reference-count": 65,
"references-count": 65,
"relation": {},
"score": 1,
"short-container-title": [
"IJERPH"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Health, Toxicology and Mutagenesis",
"Public Health, Environmental and Occupational Health"
],
"subtitle": [],
"title": [
"Effect of a Nutritional Support System to Increase Survival and Reduce Mortality in Patients with COVID-19 in Stage III and Comorbidities: A Blinded Randomized Controlled Clinical Trial"
],
"type": "journal-article",
"volume": "19"
}
lealmartinez
