Effectiveness and safety of azvudine in the treatment of COVID-19 patients: a retrospective cohort study using propensity score matching
et al., Frontiers in Cellular and Infection Microbiology, doi:10.3389/fcimb.2025.1584261, Jun 2025
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.0000000041 from 40 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
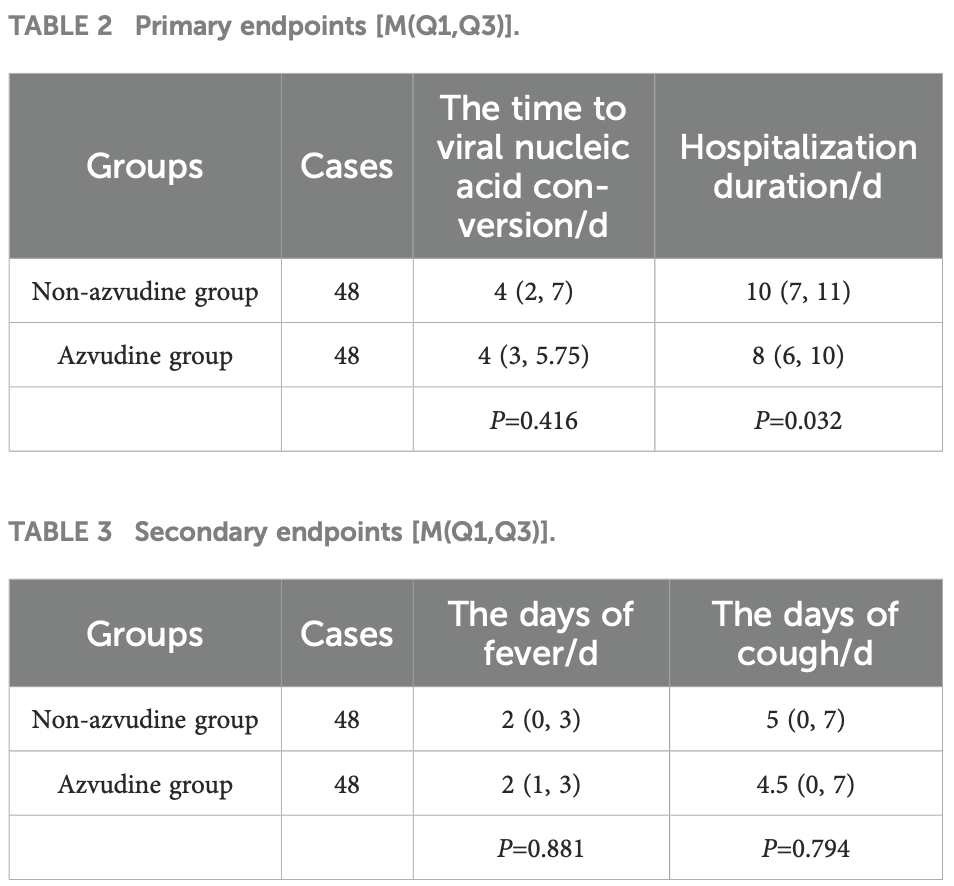
Retrospective 192 COVID-19 patients in China showing significantly shorter hospitalization with azvudine treatment, but no significant difference for viral clearance.
Viral load measured by PCR may not accurately reflect infectious virus measured by viral culture. Porter et al. show that viral load early in infection was correlated with infectious virus, but viral load late in infection could be high even with low or undetectable infectious virus. Assessing viral load later in infection may underestimate reductions in infectious virus with treatment.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments4.
|
hospitalization time, 20.0% lower, relative time 0.80, p = 0.001, treatment median 8.0 IQR 4.0 n=48, control median 10.0 IQR 4.0 n=48, propensity score matching.
|
|
time to viral-, no change, relative time 1.00, p = 1.00, treatment median 4.0 IQR 2.75 n=48, control median 4.0 IQR 5.0 n=48, propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
2.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
Zhang et al., 18 Jun 2025, retrospective, China, peer-reviewed, mean age 53.5, 6 authors, study period 1 November, 2022 - 31 December, 2022.
Contact: cndongy@163.com.
Effectiveness and safety of azvudine in the treatment of COVID-19 patients: a retrospective cohort study using propensity score matching
Frontiers in Cellular and Infection Microbiology, doi:10.3389/fcimb.2025.1584261
Background: Clinical trials have demonstrated the efficacy of azvudine in alleviating clinical symptoms among patients with coronavirus disease 2019 (COVID-19). However, evidence regarding its real-world effectiveness and safety profile remains limited. Objective: To evaluate the effectiveness and safety of azvudine in COVID-19 patients. Methods: This retrospective cohort study included 192 COVID-19 patients hospitalized in Fengtai District, Beijing, from November 1 to December 31, 2022. Patients were divided into azvudine (n=118) and non-azvudine (n=74) groups. Propensity score matching (PSM) was applied to balance baseline characteristics (age, sex, vaccination status, etc.), yielding 48 matched pairs. Outcomes included time to SARS-CoV-2 RNA negativity, hospitalization duration, and symptom resolution (fever, cough). Adverse events were recorded. Results: After PSM, 48 pairs of COVID-19 patients were identified. The azvudine group exhibited significantly shorter hospitalization than the non-azvudine group (median: 8 vs. 10 days, P ≤ 0.05). No significant differences were observed in time to RNA negativity (4.23 vs. 4.52 days, P>0.05), fever duration (2 vs. 2 days, P>0.05), or cough duration (4.5 vs. 5 days, P>0.05). One case of mild gastrointestinal discomfort was reported in the azvudine group.
Conclusion: Azvudine significantly reduced hospitalization duration in mild-tomoderate COVID-19 patients with a favorable safety profile.
Ethics statement The studies involving humans were approved by Medical Ethics Committee, General Hospital of the Chinese People's Liberation Army. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because Informed consent was waived by the ethics committee as this retrospective study analyzed anonymized clinical data without patient intervention, adhering to national guidelines for non-invasive observational research.
Author contributions
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Basoulis, Tsakanikas, Gkoufa, Bitsani, Karamanakos et al., Effectiveness of oral nirmatrelvir/ritonavir vs. Intravenous three-day remdesivir in preventing progression to severe COVID-19: A single-center, prospective, comparative, real-life study, Viruses, doi:10.3390/v15071515
Chen, Tian, Efficacy and safety of azvudine in patients with COVID-19: A systematic review and meta-analysis, Heliyon, doi:10.1016/j.heliyon.2023.e20153
Gao, Luo, Ren, Duan, Han et al., Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19, J. Infect, doi:10.1016/j.jinf.2023.03.023
Ma, Chen, The latest progress of small molecule anti-SARS-CoV-2 drugs, Chin. J. Pharmacovigilance, doi:10.19803/j.1672-8629.20230357
Maio, Lafont, Sil, Li, Bollinger et al., Fe-S cofactors in the SARS-CoV-2 RNA-dependent RNA polymerase are potential antiviral targets, Science, doi:10.1126/science.abi5224
Mazzitelli, Trunfio, Sasset, Scaglione, Ferrari et al., Risk of hospitalization and sequelae in patients with COVID-19 treated with 3day early remdesivir vs. controls in the vaccine and Omicron era: A real-life cohort study, J. Med. Virol, doi:10.1002/jmv.28660
Smith, Kalayanov, Sund, Winqvist, Maltseva et al., The design, synthesis, and antiviral activity of monofluoro and difluoro analogues of 4'-azidocytidine against hepatitis C virus replication: the discovery of 4'-azido-2'-deoxy-2'-fluorocytidine and 4'-azido-2'-dideoxy-2',2'-difluorocytidine, J. Med. Chem, doi:10.1021/jm801595c
Wang, Anirudhan, Du, Cui, Rong, RNA-dependent RNA polymerase of SARS-CoV-2 as a therapeutic target, J. Med. Virol, doi:10.1002/jmv.26264
Wei, Zeng, Wang, Gui, Zhang et al., Headto-head comparison of azvudine and nirmatrelvir/ritonavir for the hospitalized patients with COVID-19: a real-world retrospective cohort study with propensity score matching, Front. Pharmacol, doi:10.3389/fphar.2023.1274294
Xu, Yang, Zheng, Zhang, Cao et al., The pyrimidine analog FNC potently inhibits the replication of multiple enteroviruses, J. Virol, doi:10.1128/JVI.00204-20
Yu, Chang, The first Chinese oral anti-COVID-19 drug Azvudine launched, Innovation (Camb), doi:10.1016/j.xinn.2022.100321
Yu, Qi, Fang, Zhang, Chen et al., Efficacy of jinyebaidu granule and lianhuaqingwen capsule in the treatment of severe COVID-19 patients:A retrospective cohort study adjusted with propensity score matching, Pharmacol. Clinics Chin. Materia Med, doi:10.13412/j.cnki.zyyl.20220608.004
Yuan, Jiao, Qu, Yang, Liu, The development of COVID-19 treatment, Front. Immunol, doi:10.3389/fimmu.2023.1125246
Zhang, Li, Qian, Xu, Clinical effectiveness evaluation of Azvudine in mild and moderate high-risk patients with COVID-19 infection, Chin. J. Hosp. Pharm
Zhang, Li, Wang, Liu, Lu et al., Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduct Target Ther, doi:10.1038/s41392-021-00835-6
Zhou, Zhang, Yang, Zhao, Zheng et al., Novel nucleoside analogue FNC is effective against both wild-type and lamivudine-resistant HBV clinical isolates, Antivir Ther, doi:10.3851/IMP2292
Zhu, Efficacy and safety evaluation of Azvudine in the prospective treatment of COVID-19 based on four phase III clinical trials, Front. Pharmacol, doi:10.3389/fphar.2023.1228548
DOI record:
{
"DOI": "10.3389/fcimb.2025.1584261",
"ISSN": [
"2235-2988"
],
"URL": "http://dx.doi.org/10.3389/fcimb.2025.1584261",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>Clinical trials have demonstrated the efficacy of azvudine in alleviating clinical symptoms among patients with coronavirus disease 2019 (COVID-19). However, evidence regarding its real-world effectiveness and safety profile remains limited.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To evaluate the effectiveness and safety of azvudine in COVID-19 patients.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>This retrospective cohort study included 192 COVID-19 patients hospitalized in Fengtai District, Beijing, from November 1 to December 31, 2022. Patients were divided into azvudine (n=118) and non-azvudine (n=74) groups. Propensity score matching (PSM) was applied to balance baseline characteristics (age, sex, vaccination status, etc.), yielding 48 matched pairs. Outcomes included time to SARS-CoV-2 RNA negativity, hospitalization duration, and symptom resolution (fever, cough). Adverse events were recorded.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>After PSM, 48 pairs of COVID-19 patients were identified. The azvudine group exhibited significantly shorter hospitalization than the non-azvudine group (median: 8 vs. 10 days, <jats:italic>P ≤</jats:italic> 0.05). No significant differences were observed in time to RNA negativity (4.23 vs. 4.52 days, <jats:italic>P</jats:italic>&gt;0.05), fever duration (2 vs. 2 days, <jats:italic>P</jats:italic>&gt;0.05), or cough duration (4.5 vs. 5 days, <jats:italic>P</jats:italic>&gt;0.05). One case of mild gastrointestinal discomfort was reported in the azvudine group.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Azvudine significantly reduced hospitalization duration in mild-to-moderate COVID-19 patients with a favorable safety profile.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fcimb.2025.1584261"
],
"author": [
{
"affiliation": [],
"family": "Zhang",
"given": "Jing",
"sequence": "first"
},
{
"affiliation": [],
"family": "Wang",
"given": "Fang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xie",
"given": "Ying",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Qianyu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Zhenzhen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dong",
"given": "Yuan",
"sequence": "additional"
}
],
"container-title": "Frontiers in Cellular and Infection Microbiology",
"container-title-short": "Front. Cell. Infect. Microbiol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2025,
6,
18
]
],
"date-time": "2025-06-18T05:41:50Z",
"timestamp": 1750225310000
},
"deposited": {
"date-parts": [
[
2025,
6,
18
]
],
"date-time": "2025-06-18T05:41:53Z",
"timestamp": 1750225313000
},
"indexed": {
"date-parts": [
[
2025,
6,
19
]
],
"date-time": "2025-06-19T04:13:56Z",
"timestamp": 1750306436095,
"version": "3.41.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
6,
18
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
6,
18
]
],
"date-time": "2025-06-18T00:00:00Z",
"timestamp": 1750204800000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fcimb.2025.1584261/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2025,
6,
18
]
]
},
"published-online": {
"date-parts": [
[
2025,
6,
18
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.3390/v15071515",
"article-title": "Effectiveness of oral nirmatrelvir/ritonavir vs. Intravenous three-day remdesivir in preventing progression to severe COVID-19: A single-center, prospective, comparative, real-life study",
"author": "Basoulis",
"doi-asserted-by": "publisher",
"journal-title": "Viruses.",
"key": "B1",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1016/j.heliyon.2023.e20153",
"article-title": "Efficacy and safety of azvudine in patients with COVID-19: A systematic review and meta-analysis",
"author": "Chen",
"doi-asserted-by": "publisher",
"journal-title": "Heliyon",
"key": "B2",
"volume": "9",
"year": "2023"
},
{
"key": "B3",
"unstructured": "Circular on the issuance of a protocol for the diagnosis and treatment of novel coronavirus infections (Trial 10th edition)\n \n 2023"
},
{
"DOI": "10.1016/j.jinf.2023.03.023",
"article-title": "Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19",
"author": "Gao",
"doi-asserted-by": "publisher",
"first-page": "e158",
"journal-title": "J. Infect.",
"key": "B4",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.19803/j.1672-8629.20230357",
"article-title": "The latest progress of small molecule anti-SARS-CoV-2 drugs",
"author": "Ma",
"doi-asserted-by": "publisher",
"first-page": "961",
"journal-title": "Chin. J. Pharmacovigilance",
"key": "B5",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.1126/science.abi5224",
"article-title": "Fe-S cofactors in the SARS-CoV-2 RNA-dependent RNA polymerase are potential antiviral targets",
"author": "Maio",
"doi-asserted-by": "publisher",
"first-page": "236",
"journal-title": "Science.",
"key": "B6",
"volume": "373",
"year": "2021"
},
{
"DOI": "10.1002/jmv.28660",
"article-title": "Risk of hospitalization and sequelae in patients with COVID-19 treated with 3-day early remdesivir vs. controls in the vaccine and Omicron era: A real-life cohort study",
"author": "Mazzitelli",
"doi-asserted-by": "publisher",
"journal-title": "J. Med. Virol.",
"key": "B7",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1021/jm801595c",
"article-title": "The design, synthesis, and antiviral activity of monofluoro and difluoro analogues of 4’-azidocytidine against hepatitis C virus replication: the discovery of 4’-azido-2’-deoxy-2’-fluorocytidine and 4’-azido-2’-dideoxy-2’,2’-difluorocytidine",
"author": "Smith",
"doi-asserted-by": "publisher",
"first-page": "2971",
"journal-title": "J. Med. Chem.",
"key": "B8",
"volume": "52",
"year": "2009"
},
{
"DOI": "10.1002/jmv.26264",
"article-title": "RNA-dependent RNA polymerase of SARS-CoV-2 as a therapeutic target",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "300",
"journal-title": "J. Med. Virol.",
"key": "B9",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2023.1274294",
"article-title": "Head-to-head comparison of azvudine and nirmatrelvir/ritonavir for the hospitalized patients with COVID-19: a real-world retrospective cohort study with propensity score matching",
"author": "Wei",
"doi-asserted-by": "publisher",
"journal-title": "Front. Pharmacol.",
"key": "B10",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1128/JVI.00204-20",
"article-title": "The pyrimidine analog FNC potently inhibits the replication of multiple enteroviruses",
"author": "Xu",
"doi-asserted-by": "publisher",
"first-page": "e00204",
"journal-title": "J. Virol.",
"key": "B11",
"volume": "94",
"year": "2020"
},
{
"DOI": "10.1016/j.xinn.2022.100321",
"article-title": "The first Chinese oral anti-COVID-19 drug Azvudine launched",
"author": "Yu",
"doi-asserted-by": "publisher",
"journal-title": "Innovation (Camb).",
"key": "B12",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.13412/j.cnki.zyyl.20220608.004",
"article-title": "Efficacy of jinyebaidu granule and lianhuaqingwen capsule in the treatment of severe COVID-19 patients:A retrospective cohort study adjusted with propensity score matching",
"author": "Yu",
"doi-asserted-by": "publisher",
"first-page": "66",
"journal-title": "Pharmacol. Clinics Chin. Materia Med.",
"key": "B13",
"volume": "39",
"year": "2023"
},
{
"DOI": "10.3389/fimmu.2023.1125246",
"article-title": "The development of COVID-19 treatment",
"author": "Yuan",
"doi-asserted-by": "publisher",
"journal-title": "Front. Immunol.",
"key": "B14",
"volume": "14",
"year": "2023"
},
{
"article-title": "Clinical effectiveness evaluation of Azvudine in mild and moderate high-risk patients with COVID-19 infection",
"author": "Zhang",
"journal-title": "Chin. J. Hosp. Pharm.",
"key": "B15",
"volume": "43",
"year": "2023"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"article-title": "Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "414",
"journal-title": "Signal Transduct Target Ther.",
"key": "B16",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.3851/IMP2292",
"article-title": "Novel nucleoside analogue FNC is effective against both wild-type and lamivudine-resistant HBV clinical isolates",
"author": "Zhou",
"doi-asserted-by": "publisher",
"first-page": "1593",
"journal-title": "Antivir Ther.",
"key": "B17",
"volume": "17",
"year": "2012"
},
{
"DOI": "10.3389/fphar.2023.1228548",
"article-title": "Efficacy and safety evaluation of Azvudine in the prospective treatment of COVID-19 based on four phase III clinical trials",
"author": "Zhu",
"doi-asserted-by": "publisher",
"journal-title": "Front. Pharmacol.",
"key": "B18",
"volume": "14",
"year": "2023"
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fcimb.2025.1584261/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effectiveness and safety of azvudine in the treatment of COVID-19 patients: a retrospective cohort study using propensity score matching",
"type": "journal-article",
"update-policy": "https://doi.org/10.3389/crossmark-policy",
"volume": "15"
}
