Head-to-head comparison of azvudine and nirmatrelvir/ritonavir for the hospitalized patients with COVID-19: a real-world retrospective cohort study with propensity score matching
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2023.1274294, Oct 2023
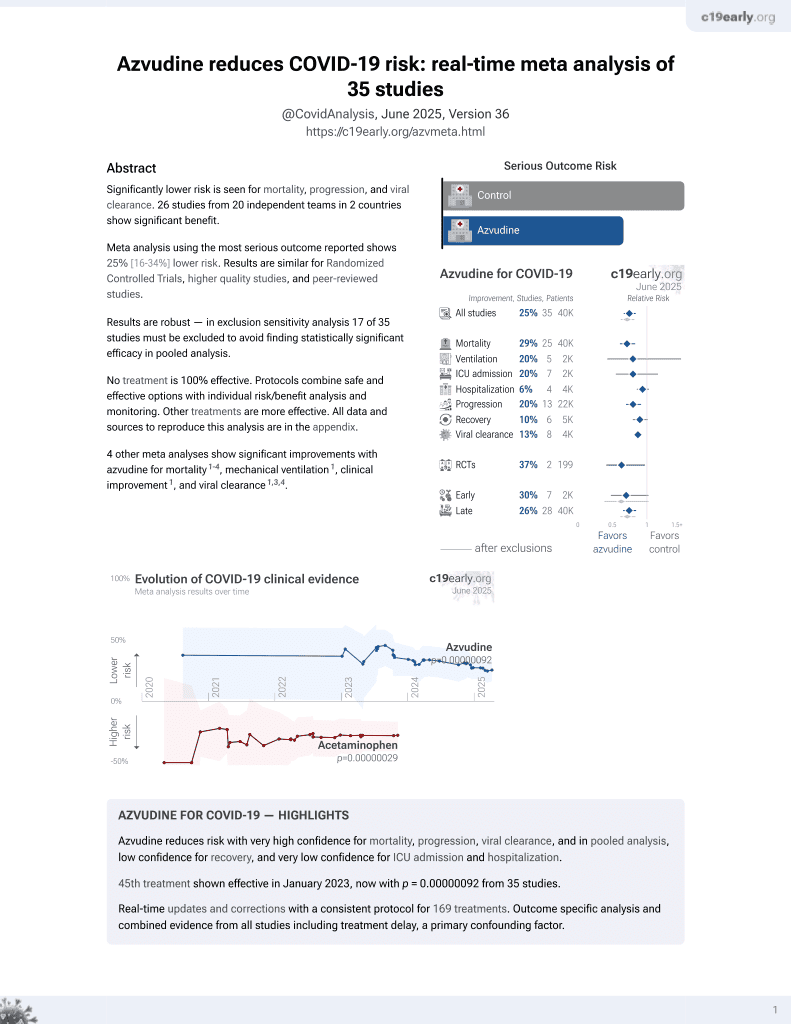
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
PSM retrospective 725 hospitalized COVID-19 patients in China compared the effectiveness and safety of the oral antivirals azvudine and paxlovid. There was no significant difference in the risk of disease progression between groups, but azvudine was associated with lower ICU admission and invasive ventilation use.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
Study covers paxlovid and azvudine.
|
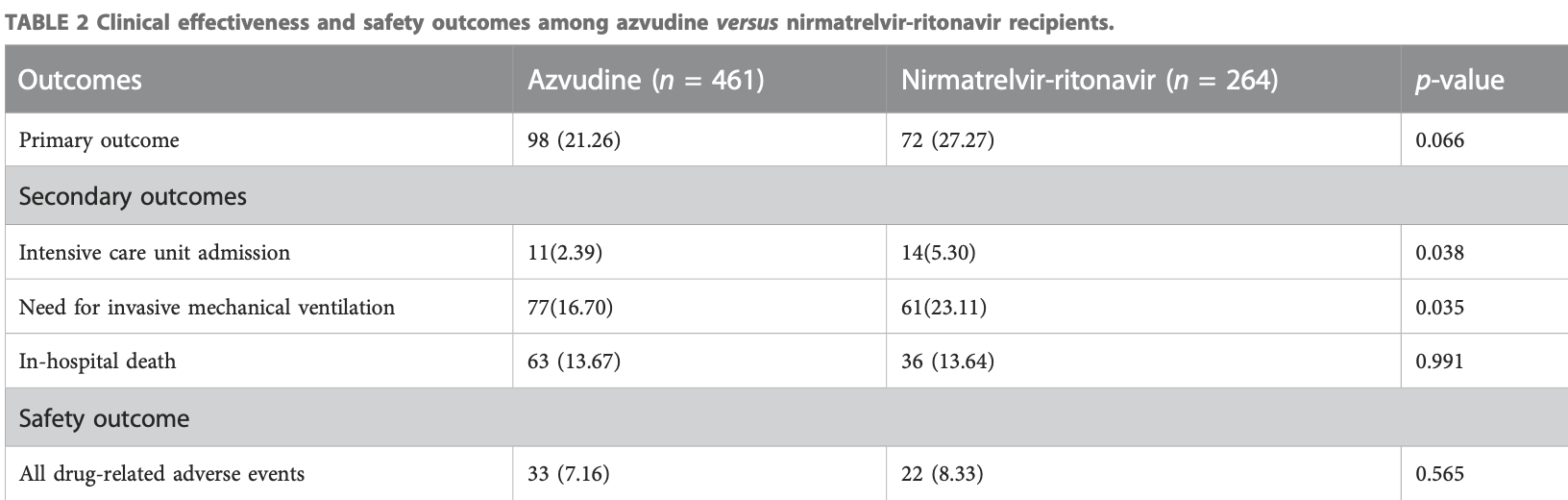
risk of death, 0.2% higher, RR 1.00, p = 1.00, treatment 63 of 461 (13.7%), control 36 of 264 (13.6%).
|
|
risk of mechanical ventilation, 27.7% lower, RR 0.72, p = 0.04, treatment 77 of 461 (16.7%), control 61 of 264 (23.1%), NNT 16.
|
|
risk of ICU admission, 55.0% lower, RR 0.45, p = 0.05, treatment 11 of 461 (2.4%), control 14 of 264 (5.3%), NNT 34.
|
|
risk of progression, 22.1% lower, RR 0.78, p = 0.07, treatment 98 of 461 (21.3%), control 72 of 264 (27.3%), NNT 17, ICU admission, invasive mechanical ventilation, and in-hospital death, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Wei et al., 13 Oct 2023, retrospective, China, peer-reviewed, 8 authors, study period 1 December, 2022 - 31 January, 2023, this trial compares with another treatment - results may be better when compared to placebo.
Contact: 13620327@qq.com, ld_2069@163.com.
Head-to-head comparison of azvudine and nirmatrelvir/ritonavir for the hospitalized patients with COVID-19: a real-world retrospective cohort study with propensity score matching
Frontiers in Pharmacology, doi:10.3389/fphar.2023.1274294
Background: Nirmatrelvir/ritonavir and azvudine have been approved for the early treatment of COVID-19 in China, however, limited real-world data exists regarding their effectiveness and safety.
Methods: We conducted a retrospective cohort study involving the hospitalized COVID-19 patients in China between December 2022 and January 2023. Demographic, clinical, and safety variables were recorded. Results: Among the 6,616 hospitalized COVID-19 patients, we included a total of 725 patients including azvudine recipients (N = 461) and nirmatrelvir/ritonavir (N = 264) recipients after exclusions and propensity score matching (1:2). There was no significant difference in the composite disease progression events between azvudine (98, 21.26%) and nirmatrelvir/ritonavir (72, 27.27%) groups (p = 0.066). Azvudine was associated with a significant reduction in secondary outcomes, including the percentage of intensive care unit admission (p = 0.038) and the need for invasive mechanical ventilation (p = 0.035), while the in-hospital death event did not significantly differ (p = 0.991). As for safety outcomes, 33 out of 461 patients (7.16%) in azvudine group and 22 out of 264 patients (8.33%) in nirmatrelvir/ritonavir group experienced drug-related adverse events between the day of admission (p = 0.565).
Conclusion: In our real-world setting, azvudine treatment demonstrated similar safety compared to nirmatrelvir/ritonavir in hospitalized COVID-19 patients. Additionally, it showed slightly better clinical benefits in this population. However, further confirmation through additional clinical trials is necessary.
Ethics statement The studies involving humans were approved by the institutional review board of Tongji hospital (TJ-IRB20230202). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because Surveys and observational study.
Author contributions A-HW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing-original draft, Writing-review and editing. LZ: Data curation, Formal Analysis, Methodology, Writing-review and editing. LW: Formal Analysis, Methodology, Writing-original draft. LG: Investigation, Writing-review and editing. W-TZ: Investigation, Project administration, Writing-review and editing. X-PG: Project administration, Writing-review and editing. JL: Supervision, Writing-review and editing. DL: Supervision, Writing-review and editing.
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their
Supplementary material The Supplementary Material for this article can be found online at:..
References
Amani, Amani, Efficacy and safety of nirmatrelvir/ritonavir (Paxlovid) for COVID-19: a rapid review and meta-analysis, J. Med. virology, doi:10.1002/jmv.28441
Arbel, Wolff Sagy, Hoshen, Battat, Lavie et al., Nirmatrelvir use and severe covid-19 outcomes during the omicron surge, N. Engl. J. Med, doi:10.1056/NEJMoa2204919
Cheema, Jafar, Sohail, Shahid, Sahra et al., Nirmatrelvir-ritonavir for the treatment of COVID-19 patients: a systematic review and meta-analysis, J. Med. virology, doi:10.1002/jmv.28471
Chen, Wang, Chang, Antiviral therapy of coronavirus disease 2019 (COVID-19), J. Formos. Med. Assoc. = Taiwan yi zhi, doi:10.1016/j.jfma.2023.08.029
Deng, Li, Sun, Jin, Zhou et al., Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: a retrospective cohort study, J. Med. virology, doi:10.1002/jmv.28756
Gao, Luo, Ren, Duan, Han et al., Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19, J. Infect, doi:10.1016/j.jinf.2023.03.023
Gentile, Scotto, Schiano Moriello, Pinchera, Villari et al., Nirmatrelvir/ritonavir and molnupiravir in the treatment of mild/moderate COVID-19: results of a real-life study, Vaccines, doi:10.3390/vaccines10101731
Hammond, Leister-Tebbe, Gardner, Abreu, Bao et al., Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2118542
Loos, Beijnen, Schinkel, The mechanism-based inactivation of CYP3A4 by ritonavir: what mechanism?, Int. J. Mol. Sci, doi:10.3390/ijms23179866
Marzi, Vakil, Bahmanyar, Zarenezhad, Paxlovid: mechanism of action, synthesis, and in silico study, BioMed Res. Int, doi:10.1155/2022/7341493
Mazzitelli, Mengato, Sasset, Ferrari, Gardin et al., Molnupiravir and nirmatrelvir/ritonavir: tolerability, safety, and adherence in a retrospective cohort study, Viruses, doi:10.3390/v15020384
Mazzitelli, Trunfio, Sasset, Scaglione, Ferrari et al., Risk of hospitalization and sequelae in patients with COVID-19 treated with 3day early remdesivir vs. controls in the vaccine and Omicron era: a real-life cohort study, J. Med. virology, doi:10.1002/jmv.28660
Miljanovic, Cirkovic, Lazarevic, Knezevic, Cupic et al., Clinical efficacy of anti-SARS-CoV-2 monoclonal antibodies in preventing hospitalisation and mortality among patients infected with Omicron variants: a systematic review and meta-analysis, Rev. Med. virology, doi:10.1002/rmv.2439
Murakami, Hayden, Hills, Al-Samkari, Casey et al., Therapeutic advances in COVID-19, Nat. Rev. Nephrol, doi:10.1038/s41581-022-00642-4
Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients, Clin. Infect. Dis, doi:10.1093/cid/ciac443
Ren, Luo, Yu, Song, Liang et al., A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study, Adv. Sci, doi:10.1002/advs.202001435
Shen, Xiao, Sun, Li, Wu et al., Real-world effectiveness of Azvudine in hospitalized patients with COVID-19: a retrospective cohort study, medRxiv, doi:10.1101/2023.01.23.23284899
Singh, De Wit, Antiviral agents for the treatment of COVID-19: progress and challenges, Cell. Rep. Med, doi:10.1016/j.xcrm.2022.100549
Wang, Yang, Luo, Peng, Dai et al., Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro, PloS one, doi:10.1371/journal.pone.0105617
Wen, Chen, Tang, Wang, Zhou et al., Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis, Ann. Med, doi:10.1080/07853890.2022.2034936
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of early molnupiravir or nirmatrelvirritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study, Lancet. Infect. Dis, doi:10.1016/S1473-3099(22)00507-2
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among communitydwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study, Lancet, doi:10.1016/S0140-6736(22)01586-0
Yu, Chang, The first Chinese oral anti-COVID-19 drug Azvudine launched, Innov. Camb. (Mass.)), doi:10.1016/j.xinn.2022.100321
Zhang, Li, Wang, Liu, Lu et al., Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduct. Target. Ther, doi:10.1038/s41392-021-00835-6
Zheng, Ma, Wang, Cheng, Zhou et al., Efficacy and safety of Paxlovid for COVID-19:a meta-analysis, J. Infect, doi:10.1016/j.jinf.2022.09.027
Zhou, Kelly, Liang, Li, Shen et al., Realworld effectiveness of nirmatrelvir/ritonavir in preventing hospitalization among patients with COVID-19 at high risk for severe disease in the United States: a nationwide population-based cohort study, medRxiv, doi:10.1101/2022.09.13.22279908
DOI record:
{
"DOI": "10.3389/fphar.2023.1274294",
"ISSN": [
"1663-9812"
],
"URL": "http://dx.doi.org/10.3389/fphar.2023.1274294",
"abstract": "<jats:p><jats:bold>Background:</jats:bold> Nirmatrelvir/ritonavir and azvudine have been approved for the early treatment of COVID-19 in China, however, limited real-world data exists regarding their effectiveness and safety.</jats:p><jats:p><jats:bold>Methods:</jats:bold> We conducted a retrospective cohort study involving the hospitalized COVID-19 patients in China between December 2022 and January 2023. Demographic, clinical, and safety variables were recorded.</jats:p><jats:p><jats:bold>Results:</jats:bold> Among the 6,616 hospitalized COVID-19 patients, we included a total of 725 patients including azvudine recipients (N = 461) and nirmatrelvir/ritonavir (N = 264) recipients after exclusions and propensity score matching (1:2). There was no significant difference in the composite disease progression events between azvudine (98, 21.26%) and nirmatrelvir/ritonavir (72, 27.27%) groups (<jats:italic>p</jats:italic> = 0.066). Azvudine was associated with a significant reduction in secondary outcomes, including the percentage of intensive care unit admission (<jats:italic>p</jats:italic> = 0.038) and the need for invasive mechanical ventilation (<jats:italic>p</jats:italic> = 0.035), while the in-hospital death event did not significantly differ (<jats:italic>p</jats:italic> = 0.991). As for safety outcomes, 33 out of 461 patients (7.16%) in azvudine group and 22 out of 264 patients (8.33%) in nirmatrelvir/ritonavir group experienced drug-related adverse events between the day of admission (<jats:italic>p</jats:italic> = 0.565).</jats:p><jats:p><jats:bold>Conclusion:</jats:bold> In our real-world setting, azvudine treatment demonstrated similar safety compared to nirmatrelvir/ritonavir in hospitalized COVID-19 patients. Additionally, it showed slightly better clinical benefits in this population. However, further confirmation through additional clinical trials is necessary.</jats:p>",
"alternative-id": [
"10.3389/fphar.2023.1274294"
],
"author": [
{
"affiliation": [],
"family": "Wei",
"given": "An-Hua",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zeng",
"given": "Lu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Lu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gui",
"given": "Lin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Wen-Ting",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gong",
"given": "Xue-Peng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Dong",
"sequence": "additional"
}
],
"container-title": "Frontiers in Pharmacology",
"container-title-short": "Front. Pharmacol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
10,
13
]
],
"date-time": "2023-10-13T05:02:57Z",
"timestamp": 1697173377000
},
"deposited": {
"date-parts": [
[
2023,
10,
13
]
],
"date-time": "2023-10-13T05:03:00Z",
"timestamp": 1697173380000
},
"indexed": {
"date-parts": [
[
2023,
10,
14
]
],
"date-time": "2023-10-14T11:46:17Z",
"timestamp": 1697283977332
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
10,
13
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
13
]
],
"date-time": "2023-10-13T00:00:00Z",
"timestamp": 1697155200000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2023.1274294/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
10,
13
]
]
},
"published-online": {
"date-parts": [
[
2023,
10,
13
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1002/jmv.28441",
"article-title": "Efficacy and safety of nirmatrelvir/ritonavir (Paxlovid) for COVID-19: a rapid review and meta-analysis",
"author": "Amani",
"doi-asserted-by": "publisher",
"first-page": "e28441",
"journal-title": "J. Med. virology",
"key": "B1",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2204919",
"article-title": "Nirmatrelvir use and severe covid-19 outcomes during the omicron surge",
"author": "Arbel",
"doi-asserted-by": "publisher",
"first-page": "790",
"journal-title": "N. Engl. J. Med.",
"key": "B2",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1002/jmv.28471",
"article-title": "Nirmatrelvir-ritonavir for the treatment of COVID-19 patients: a systematic review and meta-analysis",
"author": "Cheema",
"doi-asserted-by": "publisher",
"first-page": "e28471",
"journal-title": "J. Med. virology",
"key": "B3",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1016/j.jfma.2023.08.029",
"article-title": "Antiviral therapy of coronavirus disease 2019 (COVID-19)",
"author": "Chen",
"doi-asserted-by": "publisher",
"journal-title": "J. Formos. Med. Assoc. = Taiwan yi zhi.",
"key": "B4",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28756",
"article-title": "Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: a retrospective cohort study",
"author": "Deng",
"doi-asserted-by": "publisher",
"first-page": "e28756",
"journal-title": "J. Med. virology",
"key": "B5",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2023.03.023",
"article-title": "Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19",
"author": "Gao",
"doi-asserted-by": "publisher",
"first-page": "e158",
"journal-title": "J. Infect.",
"key": "B6",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.3390/vaccines10101731",
"article-title": "Nirmatrelvir/ritonavir and molnupiravir in the treatment of mild/moderate COVID-19: results of a real-life study",
"author": "Gentile",
"doi-asserted-by": "publisher",
"first-page": "1731",
"journal-title": "Vaccines",
"key": "B7",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19",
"author": "Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"journal-title": "N. Engl. J. Med.",
"key": "B8",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.3390/ijms23179866",
"article-title": "The mechanism-based inactivation of CYP3A4 by ritonavir: what mechanism?",
"author": "Loos",
"doi-asserted-by": "publisher",
"first-page": "9866",
"journal-title": "Int. J. Mol. Sci.",
"key": "B9",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1155/2022/7341493",
"article-title": "Paxlovid: mechanism of action, synthesis, and in silico study",
"author": "Marzi",
"doi-asserted-by": "publisher",
"first-page": "7341493",
"journal-title": "BioMed Res. Int.",
"key": "B10",
"volume": "2022",
"year": "2022"
},
{
"DOI": "10.1002/jmv.28660",
"article-title": "Risk of hospitalization and sequelae in patients with COVID-19 treated with 3-day early remdesivir vs. controls in the vaccine and Omicron era: a real-life cohort study",
"author": "Mazzitelli",
"doi-asserted-by": "publisher",
"first-page": "e28660",
"journal-title": "J. Med. virology",
"key": "B11",
"volume": "95",
"year": ""
},
{
"DOI": "10.3390/v15020384",
"article-title": "Molnupiravir and nirmatrelvir/ritonavir: tolerability, safety, and adherence in a retrospective cohort study",
"author": "Mazzitelli",
"doi-asserted-by": "publisher",
"first-page": "384",
"journal-title": "Viruses",
"key": "B12",
"volume": "15",
"year": ""
},
{
"DOI": "10.1002/rmv.2439",
"article-title": "Clinical efficacy of anti-SARS-CoV-2 monoclonal antibodies in preventing hospitalisation and mortality among patients infected with Omicron variants: a systematic review and meta-analysis",
"author": "Miljanovic",
"doi-asserted-by": "publisher",
"first-page": "e2439",
"journal-title": "Rev. Med. virology",
"key": "B13",
"volume": "33",
"year": "2023"
},
{
"DOI": "10.1038/s41581-022-00642-4",
"article-title": "Therapeutic advances in COVID-19",
"author": "Murakami",
"doi-asserted-by": "publisher",
"first-page": "38",
"journal-title": "Nat. Rev. Nephrol.",
"key": "B14",
"volume": "19",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac443",
"article-title": "Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients",
"author": "Najjar-Debbiny",
"doi-asserted-by": "publisher",
"first-page": "e342",
"journal-title": "Clin. Infect. Dis.",
"key": "B15",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1002/advs.202001435",
"article-title": "A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study",
"author": "Ren",
"doi-asserted-by": "publisher",
"first-page": "e2001435",
"journal-title": "Adv. Sci. (Weinheim, Baden-Wurttemberg, Ger.",
"key": "B16",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1101/2023.01.23.23284899",
"article-title": "Real-world effectiveness of Azvudine in hospitalized patients with COVID-19: a retrospective cohort study",
"author": "Shen",
"doi-asserted-by": "publisher",
"key": "B17",
"year": "2023"
},
{
"DOI": "10.1016/j.xcrm.2022.100549",
"article-title": "Antiviral agents for the treatment of COVID-19: progress and challenges",
"author": "Singh",
"doi-asserted-by": "publisher",
"first-page": "100549",
"journal-title": "Cell. Rep. Med.",
"key": "B18",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0105617",
"article-title": "Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "e105617",
"journal-title": "PloS one",
"key": "B19",
"volume": "9",
"year": "2014"
},
{
"DOI": "10.1080/07853890.2022.2034936",
"article-title": "Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis",
"author": "Wen",
"doi-asserted-by": "publisher",
"first-page": "516",
"journal-title": "Ann. Med.",
"key": "B20",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)01586-0",
"article-title": "Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study",
"author": "Wong",
"doi-asserted-by": "publisher",
"first-page": "1213",
"journal-title": "Lancet (London, Engl.",
"key": "B21",
"volume": "400",
"year": ""
},
{
"DOI": "10.1016/S1473-3099(22)00507-2",
"article-title": "Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study",
"author": "Wong",
"doi-asserted-by": "publisher",
"first-page": "1681",
"journal-title": "Lancet. Infect. Dis.",
"key": "B22",
"volume": "22",
"year": ""
},
{
"DOI": "10.1016/j.xinn.2022.100321",
"article-title": "The first Chinese oral anti-COVID-19 drug Azvudine launched",
"author": "Yu",
"doi-asserted-by": "publisher",
"first-page": "100321",
"journal-title": "Innov. Camb. (Mass.))",
"key": "B23",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"article-title": "Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "414",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "B24",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/j.jinf.2022.09.027",
"article-title": "Efficacy and safety of Paxlovid for COVID-19:a meta-analysis",
"author": "Zheng",
"doi-asserted-by": "publisher",
"first-page": "66",
"journal-title": "J. Infect.",
"key": "B25",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.1101/2022.09.13.22279908",
"article-title": "Real-world effectiveness of nirmatrelvir/ritonavir in preventing hospitalization among patients with COVID-19 at high risk for severe disease in the United States: a nationwide population-based cohort study",
"author": "Zhou",
"doi-asserted-by": "publisher",
"key": "B26",
"year": "2022"
}
],
"reference-count": 26,
"references-count": 26,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2023.1274294/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology"
],
"subtitle": [],
"title": "Head-to-head comparison of azvudine and nirmatrelvir/ritonavir for the hospitalized patients with COVID-19: a real-world retrospective cohort study with propensity score matching",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "14"
}