Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients
et al., Signal Transduction and Targeted Therapy, doi:10.1038/s41392-021-00835-6, Dec 2021
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.0000000041 from 40 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
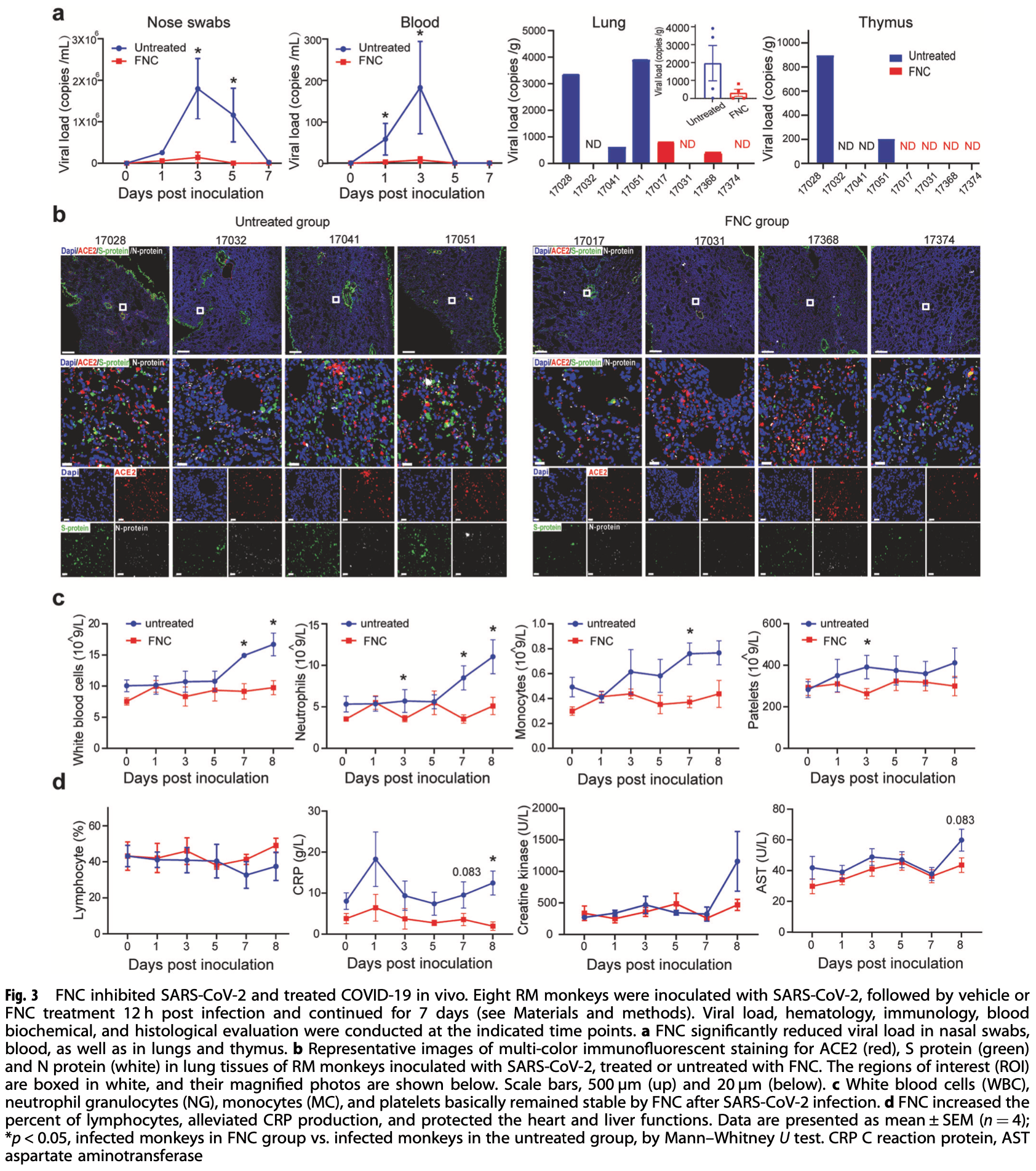
Analysis of azvudine (FNC) as a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Authors found that FNC inhibited SARS-CoV-2 and HCoV-OC43 coronavirus replication in vitro with EC50 between 1.2 and 4.3 μM. Oral FNC in rats showed substantial concentration of the active triphosphate form in the thymus and peripheral blood mononuclear cells. In SARS-CoV-2 infected rhesus macaques, FNC treatment reduced viral load, recuperated the thymus, improved lymphocyte profiles, alleviated inflammation and organ damage, and reduced ground-glass opacities in chest x-ray. Single-cell sequencing suggested FNC promoted thymus function. In a clinical trial of 31 COVID-19 patients, oral FNC resulted in 100% viral RNA negative conversion in 3.29 ± 2.22 days and 100% hospital discharge in 9.00 ± 4.93 days, with only minor side effects.
Zhang et al., 6 Dec 2021, prospective, China, peer-reviewed, 29 authors.
Contact: pengxiaozhong@pumc.edu.cn, chzs1990@163.com, changjunbiao@zzu.edu.cn, jiang.jdong@163.com.
Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients
Signal Transduction and Targeted Therapy, doi:10.1038/s41392-021-00835-6
Azvudine (FNC) is a nucleoside analog that inhibits HIV-1 RNA-dependent RNA polymerase (RdRp). Recently, we discovered FNC an agent against SARS-CoV-2, and have taken it into Phase III trial for COVID-19 patients. FNC monophosphate analog inhibited SARS-CoV-2 and HCoV-OC43 coronavirus with an EC 50 between 1.2 and 4.3 μM, depending on viruses or cells, and selective index (SI) in 15-83 range. Oral administration of FNC in rats revealed a substantial thymus-homing feature, with FNC triphosphate (the active form) concentrated in the thymus and peripheral blood mononuclear cells (PBMC). Treating SARS-CoV-2 infected rhesus macaques with FNC (0.07 mg/kg, qd, orally) reduced viral load, recuperated the thymus, improved lymphocyte profiles, alleviated inflammation and organ damage, and lessened ground-glass opacities in chest X-ray. Single-cell sequencing suggested the promotion of thymus function by FNC. A randomized, single-arm clinical trial of FNC on compassionate use (n = 31) showed that oral FNC (5 mg, qd) cured all COVID-19 patients, with 100% viral ribonucleic acid negative conversion in 3.29 ± 2.22 days (range: 1-9 days) and 100% hospital discharge rate in 9.00 ± 4.93 days (range: 2-25 days). The side-effect of FNC is minor and transient dizziness and nausea in 16.12% (5/31) patients. Thus, FNC might cure COVID-19 through its anti-SARS-CoV-2 activity concentrated in the thymus, followed by promoted immunity.
AUTHOR CONTRIBUTIONS
ADDITIONAL INFORMATION Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41392-021-00835-6. Competing interests: The authors declare no competing interests.
References
Bert, SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls, Nature
Cao, A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19, N. Engl. J. Med
Channappanavar, Zhao, Perlman, T cell-mediated immune response to respiratory coronaviruses, Immunol. Res
Chau, The longitudinal immune response to coronavirus disease 2019: chasing the cytokine storm, Arthritis Rheumatol
Chen, Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019, Clin. Infect. Dis
Chen, Liu, Guo, Emerging coronaviruses: genome structure, replication, and pathogenesis, J. Med. Virol
Cluitmans, Esendam, Landegent, Willemze, Falkenburg, IL-4 down-regulates IL-2-, IL-3-, and GM-CSF-induced cytokine gene expression in peripheral blood monocytes, Ann. Hematol
Costela-Ruiz, Illescas-Montes, Puerta-Puerta, Ruiz, Melguizo-Rodríguez, SARS-CoV-2 infection: the role of cytokines in COVID-19 disease, Cytokine Growth Factor Rev
Giamarellos-Bourboulis, Complex immune dysregulation in COVID-19 patients with severe respiratory failure, Cell Host Microbe
Grein, Compassionate use of remdesivir for patients with severe covid-19, N. Engl. J. Med
Grifoni, Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals, Cell
Guan, Clinical characteristics of coronavirus disease 2019 in China, N. Engl. J. Med
Gubernatorova, Gorshkova, Polinova, Drutskaya, IL-6: relevance for immunopathology of SARS-CoV-2, Cytokine Growth Factor Rev
Himmelrich, Parra-Lopez, Tacchini-Cottier, Louis, Launois, The IL-4 rapidly produced in BALB/c mice after infection with Leishmania major down-regulates IL-12 receptor beta 2-chain expression on CD4+ T cells resulting in a state of unresponsiveness to IL-12, J. Immunol
Huang, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Ip, Hoshi, Shouval, Snapper, Medzhitov, Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages, Science
Kaneko, Loss of Bcl-6-expressing T follicular helper cells and germinal centers in COVID-19, Cell
Klumpp, 2′-deoxy-4′-azido nucleoside analogs are highly potent inhibitors of hepatitis C virus replication despite the lack of 2′-α-hydroxyl groups*, J. Biol. Chem
Le, Single-cell RNA-Seq mapping of human thymopoiesis reveals lineage specification trajectories and a commitment spectrum in T cell development, Immunity
Lescure, Clinical and virological data of the first cases of COVID-19 in Europe: a case series, Lancet Infect. Dis
Leyva-Castillo, Mast cell-derived IL-13 downregulates IL-12 production by skin dendritic cells to inhibit the TH1 cell response to cutaneous antigen exposure, J. Allergy Clin. Immunol
Linker-Israeli, Exogenous IL-10 and IL-4 down-regulate IL-6 production by SLE-derived PBMC, Clin. Immunol
Liu, Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients, EBioMedicine
Lu, Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding, Lancet
Martinez, Compounds with therapeutic potential against novel respiratory 2019 coronavirus, Antimicrob. Agents Chemother
Munster, Respiratory disease in rhesus macaques inoculated with SARS-CoV-2, Nature
Napoli, Immune reactivity during COVID-19: Implications for treatment, Immunol. Lett
Rahi, Hematologic disorders associated with COVID-19: a review, Ann. Hematol
Ren, A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study, Adv. Sci
Ren, COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas, Cell
Shukla, poly(UG)-tailed RNAs in genome protection and epigenetic inheritance, Nature
Sun, Risk factors for mortality in 244 older adults with COVID-19 in Wuhan, China: a retrospective study, J. Am. Geriatr. Soc
Terpos, Hematological findings and complications of COVID-19, Am. J. Hematol
Tian, Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study, Lancet Oncol
Van Der Waart, Van Der Velden, Blijlevens, Dolstra, Targeting the IL17 pathway for the prevention of graft-versus-host disease, Biol. Blood Marrow Transplant
Wang, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, J. Am. Med. Assoc
Wang, Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2, Cell
Wang, Remdesivir in adults with severe COVID-19: a randomised, doubleblind, placebo-controlled, multicentre trial, Lancet
Wang, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro, PloS ONE
Williamson, Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2, Nature
Xu, The pyrimidine analog FNC potently inhibits the replication of multiple enteroviruses, J. Virol
Yousefi, A global treatments for coronaviruses including COVID-19, J. Cell. Physiol
Zeng, Single-cell RNA sequencing resolves spatiotemporal development of pre-thymic lymphoid progenitors and thymus organogenesis in human embryos, Immunity
Zhang, Single-cell landscape of immunological responses in patients with COVID-19, Nat. Immunol
Zhang, The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China, Clin. Immunol
Zhang, Viral and host factors related to the clinical outcome of COVID-19, Nature
Zhao, Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis, Int. J. Infect. Dis
Zhou, A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature
Zhou, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.1038/s41392-021-00835-6",
"ISSN": [
"2059-3635"
],
"URL": "http://dx.doi.org/10.1038/s41392-021-00835-6",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Azvudine (FNC) is a nucleoside analog that inhibits HIV-1 RNA-dependent RNA polymerase (RdRp). Recently, we discovered FNC an agent against SARS-CoV-2, and have taken it into Phase III trial for COVID-19 patients. FNC monophosphate analog inhibited SARS-CoV-2 and HCoV-OC43 coronavirus with an EC<jats:sub>50</jats:sub> between 1.2 and 4.3 μM, depending on viruses or cells, and selective index (SI) in 15–83 range. Oral administration of FNC in rats revealed a substantial thymus-homing feature, with FNC triphosphate (the active form) concentrated in the thymus and peripheral blood mononuclear cells (PBMC). Treating SARS-CoV-2 infected rhesus macaques with FNC (0.07 mg/kg, qd, orally) reduced viral load, recuperated the thymus, improved lymphocyte profiles, alleviated inflammation and organ damage, and lessened ground-glass opacities in chest X-ray. Single-cell sequencing suggested the promotion of thymus function by FNC. A randomized, single-arm clinical trial of FNC on compassionate use (<jats:italic>n</jats:italic> = 31) showed that oral FNC (5 mg, qd) cured all COVID-19 patients, with 100% viral ribonucleic acid negative conversion in 3.29 ± 2.22 days (range: 1–9 days) and 100% hospital discharge rate in 9.00 ± 4.93 days (range: 2–25 days). The side-effect of FNC is minor and transient dizziness and nausea in 16.12% (5/31) patients. Thus, FNC might cure COVID-19 through its anti-SARS-CoV-2 activity concentrated in the thymus, followed by promoted immunity.</jats:p>",
"alternative-id": [
"835"
],
"article-number": "414",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "5 September 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Revised",
"name": "revised",
"order": 2,
"value": "31 October 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 3,
"value": "14 November 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 4,
"value": "6 December 2021"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Zhang",
"given": "Jin-Lan",
"sequence": "first"
},
{
"affiliation": [],
"family": "Li",
"given": "Yu-Huan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Lu-Lu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Hong-Qi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Shuai-Yao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Yong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Ke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Bin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Su-Yun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shao",
"given": "Feng-Min",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Kun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sheng",
"given": "Ning",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Rui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cui",
"given": "Jin-Jin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sun",
"given": "Pei-Chun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ma",
"given": "Chun-Xia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Bo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Zhe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wan",
"given": "Yuan-Hao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yu",
"given": "Shi-Shan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Che",
"given": "Yongsheng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Chao-Yang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Chen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Qiangqian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Li-Min",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peng",
"given": "Xiao-Zhong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheng",
"given": "Zhenshun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chang",
"given": "Jun-Biao",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1173-685X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jiang",
"given": "Jian-Dong",
"sequence": "additional"
}
],
"container-title": "Signal Transduction and Targeted Therapy",
"container-title-short": "Sig Transduct Target Ther",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
12,
6
]
],
"date-time": "2021-12-06T08:02:47Z",
"timestamp": 1638777767000
},
"deposited": {
"date-parts": [
[
2023,
2,
10
]
],
"date-time": "2023-02-10T07:06:59Z",
"timestamp": 1676012819000
},
"indexed": {
"date-parts": [
[
2024,
8,
19
]
],
"date-time": "2024-08-19T04:28:08Z",
"timestamp": 1724041688354
},
"is-referenced-by-count": 112,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
12,
6
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
6
]
],
"date-time": "2021-12-06T00:00:00Z",
"timestamp": 1638748800000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
6
]
],
"date-time": "2021-12-06T00:00:00Z",
"timestamp": 1638748800000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41392-021-00835-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41392-021-00835-6",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41392-021-00835-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2021,
12,
6
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
6
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1002/jmv.25681",
"author": "Y Chen",
"doi-asserted-by": "publisher",
"first-page": "418",
"journal-title": "J. Med. Virol.",
"key": "835_CR1",
"unstructured": "Chen, Y., Liu, Q. & Guo, D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 92, 418–423 (2020).",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30251-8",
"author": "R Lu",
"doi-asserted-by": "publisher",
"first-page": "565",
"journal-title": "Lancet",
"key": "835_CR2",
"unstructured": "Lu, R. et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395, 565–574 (2020).",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"author": "P Zhou",
"doi-asserted-by": "publisher",
"first-page": "270",
"journal-title": "Nature",
"key": "835_CR3",
"unstructured": "Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020).",
"volume": "579",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"author": "W-J Guan",
"doi-asserted-by": "publisher",
"first-page": "1708",
"journal-title": "N. Engl. J. Med.",
"key": "835_CR4",
"unstructured": "Guan, W.-J. et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720 (2020).",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"author": "F Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"journal-title": "Lancet",
"key": "835_CR5",
"unstructured": "Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062 (2020).",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30200-0",
"author": "F-X Lescure",
"doi-asserted-by": "publisher",
"first-page": "697",
"journal-title": "Lancet Infect. Dis.",
"key": "835_CR6",
"unstructured": "Lescure, F.-X. et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect. Dis. 20, 697–706 (2020).",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.ebiom.2020.102763",
"author": "J Liu",
"doi-asserted-by": "publisher",
"first-page": "102763",
"journal-title": "EBioMedicine",
"key": "835_CR7",
"unstructured": "Liu, J. et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 55, 102763–102763 (2020).",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa449",
"author": "X Chen",
"doi-asserted-by": "publisher",
"first-page": "1937",
"journal-title": "Clin. Infect. Dis.",
"key": "835_CR8",
"unstructured": "Chen, X. et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin. Infect. Dis. 71, 1937–1942 (2020).",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1016/j.clim.2020.108393",
"author": "W Zhang",
"doi-asserted-by": "publisher",
"first-page": "108393",
"journal-title": "Clin. Immunol.",
"key": "835_CR9",
"unstructured": "Zhang, W. et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 214, 108393–108393 (2020).",
"volume": "214",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2355-0",
"author": "X Zhang",
"doi-asserted-by": "publisher",
"first-page": "437",
"journal-title": "Nature",
"key": "835_CR10",
"unstructured": "Zhang, X. et al. Viral and host factors related to the clinical outcome of COVID-19. Nature 583, 437–440 (2020).",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2323-8",
"author": "A Shukla",
"doi-asserted-by": "publisher",
"first-page": "283",
"journal-title": "Nature",
"key": "835_CR11",
"unstructured": "Shukla, A. et al. poly(UG)-tailed RNAs in genome protection and epigenetic inheritance. Nature 582, 283–288 (2020).",
"volume": "582",
"year": "2020"
},
{
"DOI": "10.1128/AAC.00399-20",
"author": "MA Martinez",
"doi-asserted-by": "publisher",
"first-page": "e00399",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "835_CR12",
"unstructured": "Martinez, M. A. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob. Agents Chemother. 64, e00399–00320 (2020).",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"author": "B Cao",
"doi-asserted-by": "publisher",
"first-page": "1787",
"journal-title": "N. Engl. J. Med.",
"key": "835_CR13",
"unstructured": "Cao, B. et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 382, 1787–1799 (2020).",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1002/jcp.29785",
"author": "B Yousefi",
"doi-asserted-by": "publisher",
"first-page": "9133",
"journal-title": "J. Cell. Physiol.",
"key": "835_CR14",
"unstructured": "Yousefi, B. et al. A global treatments for coronaviruses including COVID-19. J. Cell. Physiol. 235, 9133–9142 (2020).",
"volume": "235",
"year": "2020"
},
{
"DOI": "10.1074/jbc.M708929200",
"author": "K Klumpp",
"doi-asserted-by": "publisher",
"first-page": "2167",
"journal-title": "J. Biol. Chem.",
"key": "835_CR15",
"unstructured": "Klumpp, K. et al. 2′-deoxy-4′-azido nucleoside analogs are highly potent inhibitors of hepatitis C virus replication despite the lack of 2′-α-hydroxyl groups*. J. Biol. Chem. 283, 2167–2175 (2008).",
"volume": "283",
"year": "2008"
},
{
"DOI": "10.1128/JVI.00204-20",
"author": "N Xu",
"doi-asserted-by": "publisher",
"first-page": "e00204",
"journal-title": "J. Virol.",
"key": "835_CR16",
"unstructured": "Xu, N. et al. The pyrimidine analog FNC potently inhibits the replication of multiple enteroviruses. J. Virol. 94, e00204–e00220 (2020).",
"volume": "94",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0105617",
"author": "R-R Wang",
"doi-asserted-by": "publisher",
"first-page": "e105617",
"journal-title": "PloS ONE",
"key": "835_CR17",
"unstructured": "Wang, R.-R. et al. Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro. PloS ONE 9, e105617–e105617 (2014).",
"volume": "9",
"year": "2014"
},
{
"DOI": "10.1016/j.cell.2020.06.008",
"author": "H Wang",
"doi-asserted-by": "publisher",
"first-page": "713",
"journal-title": "Cell",
"key": "835_CR18",
"unstructured": "Wang, H. et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell 182, 713–721.e9 (2020).",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1007/s00277-020-04366-y",
"author": "MS Rahi",
"doi-asserted-by": "publisher",
"first-page": "309",
"journal-title": "Ann. Hematol.",
"key": "835_CR19",
"unstructured": "Rahi, M. S. et al. Hematologic disorders associated with COVID-19: a review. Ann. Hematol. 100, 309–320 (2021).",
"volume": "100",
"year": "2021"
},
{
"DOI": "10.1016/j.imlet.2021.01.001",
"author": "C Napoli",
"doi-asserted-by": "publisher",
"first-page": "28",
"journal-title": "Immunol. Lett.",
"key": "835_CR20",
"unstructured": "Napoli, C. et al. Immune reactivity during COVID-19: Implications for treatment. Immunol. Lett. 231, 28–34 (2021).",
"volume": "231",
"year": "2021"
},
{
"DOI": "10.1002/ajh.25829",
"author": "E Terpos",
"doi-asserted-by": "publisher",
"first-page": "834",
"journal-title": "Am. J. Hematol.",
"key": "835_CR21",
"unstructured": "Terpos, E. et al. Hematological findings and complications of COVID-19. Am. J. Hematol. 95, 834–847 (2020).",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1016/S1470-2045(20)30309-0",
"author": "J Tian",
"doi-asserted-by": "publisher",
"first-page": "893",
"journal-title": "Lancet Oncol.",
"key": "835_CR22",
"unstructured": "Tian, J. et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 21, 893–903 (2020).",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1016/j.chom.2020.04.009",
"author": "EJ Giamarellos-Bourboulis",
"doi-asserted-by": "publisher",
"first-page": "992",
"journal-title": "Cell Host Microbe",
"key": "835_CR23",
"unstructured": "Giamarellos-Bourboulis, E. J. et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 27, 992–1000.e1003 (2020).",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1016/j.cytogfr.2020.05.009",
"author": "EO Gubernatorova",
"doi-asserted-by": "publisher",
"first-page": "13",
"journal-title": "Cytokine Growth Factor Rev.",
"key": "835_CR24",
"unstructured": "Gubernatorova, E. O., Gorshkova, E. A., Polinova, A. I. & Drutskaya, M. S. IL-6: relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 53, 13–24 (2020).",
"volume": "53",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2324-7",
"author": "VJ Munster",
"doi-asserted-by": "publisher",
"first-page": "268",
"journal-title": "Nature",
"key": "835_CR25",
"unstructured": "Munster, V. J. et al. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature 585, 268–272 (2020).",
"volume": "585",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.08.025",
"author": "N Kaneko",
"doi-asserted-by": "publisher",
"first-page": "143",
"journal-title": "Cell",
"key": "835_CR26",
"unstructured": "Kaneko, N. et al. Loss of Bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell 183, 143–157.e113 (2020).",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2021.01.053",
"author": "X Ren",
"doi-asserted-by": "publisher",
"first-page": "1895",
"journal-title": "Cell",
"key": "835_CR27",
"unstructured": "Ren, X. et al. COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas. Cell 184, 1895–1913.e1819 (2021).",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1038/s41590-020-0762-x",
"author": "J-Y Zhang",
"doi-asserted-by": "publisher",
"first-page": "1107",
"journal-title": "Nat. Immunol.",
"key": "835_CR28",
"unstructured": "Zhang, J.-Y. et al. Single-cell landscape of immunological responses in patients with COVID-19. Nat. Immunol. 21, 1107–1118 (2020).",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1016/j.immuni.2020.05.010",
"author": "J Le",
"doi-asserted-by": "publisher",
"first-page": "1105",
"journal-title": "Immunity",
"key": "835_CR29",
"unstructured": "Le, J. et al. Single-cell RNA-Seq mapping of human thymopoiesis reveals lineage specification trajectories and a commitment spectrum in T cell development. Immunity 52, 1105–1118.e1109 (2020).",
"volume": "52",
"year": "2020"
},
{
"DOI": "10.1016/j.immuni.2019.09.008",
"author": "Y Zeng",
"doi-asserted-by": "publisher",
"first-page": "930",
"journal-title": "Immunity",
"key": "835_CR30",
"unstructured": "Zeng, Y. et al. Single-cell RNA sequencing resolves spatiotemporal development of pre-thymic lymphoid progenitors and thymus organogenesis in human embryos. Immunity 51, 930–948.e936 (2019).",
"volume": "51",
"year": "2019"
},
{
"DOI": "10.1002/advs.202001435",
"author": "Z Ren",
"doi-asserted-by": "publisher",
"first-page": "2001435",
"journal-title": "Adv. Sci.",
"key": "835_CR31",
"unstructured": "Ren, Z. et al. A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv. Sci. 7, 2001435 (2020).",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.1585",
"author": "D Wang",
"doi-asserted-by": "publisher",
"first-page": "1061",
"journal-title": "J. Am. Med. Assoc.",
"key": "835_CR32",
"unstructured": "Wang, D. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J. Am. Med. Assoc. 323, 1061–1069 (2020).",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1007/s12026-014-8534-z",
"author": "R Channappanavar",
"doi-asserted-by": "publisher",
"first-page": "118",
"journal-title": "Immunol. Res.",
"key": "835_CR33",
"unstructured": "Channappanavar, R., Zhao, J. & Perlman, S. T cell-mediated immune response to respiratory coronaviruses. Immunol. Res. 59, 118–128 (2014).",
"volume": "59",
"year": "2014"
},
{
"DOI": "10.1016/j.cell.2020.05.015",
"author": "A Grifoni",
"doi-asserted-by": "publisher",
"first-page": "1489",
"journal-title": "Cell",
"key": "835_CR34",
"unstructured": "Grifoni, A. et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 181, 1489–1501.e1415 (2020).",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.04.086",
"author": "Q Zhao",
"doi-asserted-by": "publisher",
"first-page": "131",
"journal-title": "Int. J. Infect. Dis.",
"key": "835_CR35",
"unstructured": "Zhao, Q. et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int. J. Infect. Dis. 96, 131–135 (2020).",
"volume": "96",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"author": "C Huang",
"doi-asserted-by": "publisher",
"first-page": "497",
"journal-title": "Lancet",
"key": "835_CR36",
"unstructured": "Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020).",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1111/jgs.16533",
"author": "H Sun",
"doi-asserted-by": "publisher",
"first-page": "E19",
"journal-title": "J. Am. Geriatr. Soc.",
"key": "835_CR37",
"unstructured": "Sun, H. et al. Risk factors for mortality in 244 older adults with COVID-19 in Wuhan, China: a retrospective study. J. Am. Geriatr. Soc. 68, E19–E23 (2020).",
"volume": "68",
"year": "2020"
},
{
"DOI": "10.1002/art.41526",
"author": "AS Chau",
"doi-asserted-by": "publisher",
"first-page": "23",
"journal-title": "Arthritis Rheumatol.",
"key": "835_CR38",
"unstructured": "Chau, A. S. et al. The longitudinal immune response to coronavirus disease 2019: chasing the cytokine storm. Arthritis Rheumatol. 73, 23–35 (2021).",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1016/j.cytogfr.2020.06.001",
"author": "VJ Costela-Ruiz",
"doi-asserted-by": "publisher",
"first-page": "62",
"journal-title": "Cytokine Growth Factor Rev.",
"key": "835_CR39",
"unstructured": "Costela-Ruiz, V. J., Illescas-Montes, R., Puerta-Puerta, J. M., Ruiz, C. & Melguizo-Rodríguez, L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 54, 62–75 (2020).",
"volume": "54",
"year": "2020"
},
{
"DOI": "10.1126/science.aal3535",
"author": "WKE Ip",
"doi-asserted-by": "publisher",
"first-page": "513",
"journal-title": "Science",
"key": "835_CR40",
"unstructured": "Ip, W. K. E., Hoshi, N., Shouval, D. S., Snapper, S. & Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 356, 513–519 (2017).",
"volume": "356",
"year": "2017"
},
{
"DOI": "10.1016/j.bbmt.2014.02.007",
"author": "AB van der Waart",
"doi-asserted-by": "publisher",
"first-page": "752",
"journal-title": "Biol. Blood Marrow Transplant.",
"key": "835_CR41",
"unstructured": "van der Waart, A. B., van der Velden, W. J. F. M., Blijlevens, N. M. & Dolstra, H. Targeting the IL17 pathway for the prevention of graft-versus-host disease. Biol. Blood Marrow Transplant. 20, 752–759 (2014).",
"volume": "20",
"year": "2014"
},
{
"DOI": "10.1006/clim.1998.4680",
"author": "M Linker-Israeli",
"doi-asserted-by": "publisher",
"first-page": "6",
"journal-title": "Clin. Immunol.",
"key": "835_CR42",
"unstructured": "Linker-Israeli, M. et al. Exogenous IL-10 and IL-4 down-regulate IL-6 production by SLE-derived PBMC. Clin. Immunol. 91, 6–16 (1999).",
"volume": "91",
"year": "1999"
},
{
"DOI": "10.4049/jimmunol.161.11.6156",
"author": "H Himmelrich",
"doi-asserted-by": "publisher",
"first-page": "6156-6163",
"journal-title": "J. Immunol.",
"key": "835_CR43",
"unstructured": "Himmelrich, H., Parra-Lopez, C., Tacchini-Cottier, F., Louis, J. A. & Launois, P. The IL-4 rapidly produced in BALB/c mice after infection with Leishmania major down-regulates IL-12 receptor beta 2-chain expression on CD4+ T cells resulting in a state of unresponsiveness to IL-12. J. Immunol. 161, 6156-6163 (1998).",
"volume": "161",
"year": "1998"
},
{
"DOI": "10.1016/j.jaci.2020.11.036",
"author": "JM Leyva-Castillo",
"doi-asserted-by": "publisher",
"first-page": "2305",
"journal-title": "J. Allergy Clin. Immunol.",
"key": "835_CR44",
"unstructured": "Leyva-Castillo, J. M. et al. Mast cell–derived IL-13 downregulates IL-12 production by skin dendritic cells to inhibit the TH1 cell response to cutaneous antigen exposure. J. Allergy Clin. Immunol. 147, 2305–2315.e2303 (2021).",
"volume": "147",
"year": "2021"
},
{
"DOI": "10.1007/BF01695035",
"author": "FH Cluitmans",
"doi-asserted-by": "publisher",
"first-page": "293",
"journal-title": "Ann. Hematol.",
"key": "835_CR45",
"unstructured": "Cluitmans, F. H., Esendam, B. H., Landegent, J. E., Willemze, R. & Falkenburg, J. H. IL-4 down-regulates IL-2-, IL-3-, and GM-CSF-induced cytokine gene expression in peripheral blood monocytes. Ann. Hematol. 68, 293–298 (1994).",
"volume": "68",
"year": "1994"
},
{
"DOI": "10.1056/NEJMoa2007016",
"author": "J Grein",
"doi-asserted-by": "publisher",
"first-page": "2327",
"journal-title": "N. Engl. J. Med.",
"key": "835_CR46",
"unstructured": "Grein, J. et al. Compassionate use of remdesivir for patients with severe covid-19. N. Engl. J. Med. 382, 2327–2336 (2020).",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"author": "Y Wang",
"doi-asserted-by": "publisher",
"first-page": "1569",
"journal-title": "Lancet",
"key": "835_CR47",
"unstructured": "Wang, Y. et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395, 1569–1578 (2020).",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2423-5",
"author": "BN Williamson",
"doi-asserted-by": "publisher",
"first-page": "273",
"journal-title": "Nature",
"key": "835_CR48",
"unstructured": "Williamson, B. N. et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 585, 273–276 (2020).",
"volume": "585",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2550-z",
"author": "N Le Bert",
"doi-asserted-by": "publisher",
"first-page": "457",
"journal-title": "Nature",
"key": "835_CR49",
"unstructured": "Le Bert, N. et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 584, 457–462 (2020).",
"volume": "584",
"year": "2020"
}
],
"reference-count": 49,
"references-count": 49,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41392-021-00835-6"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "6"
}
