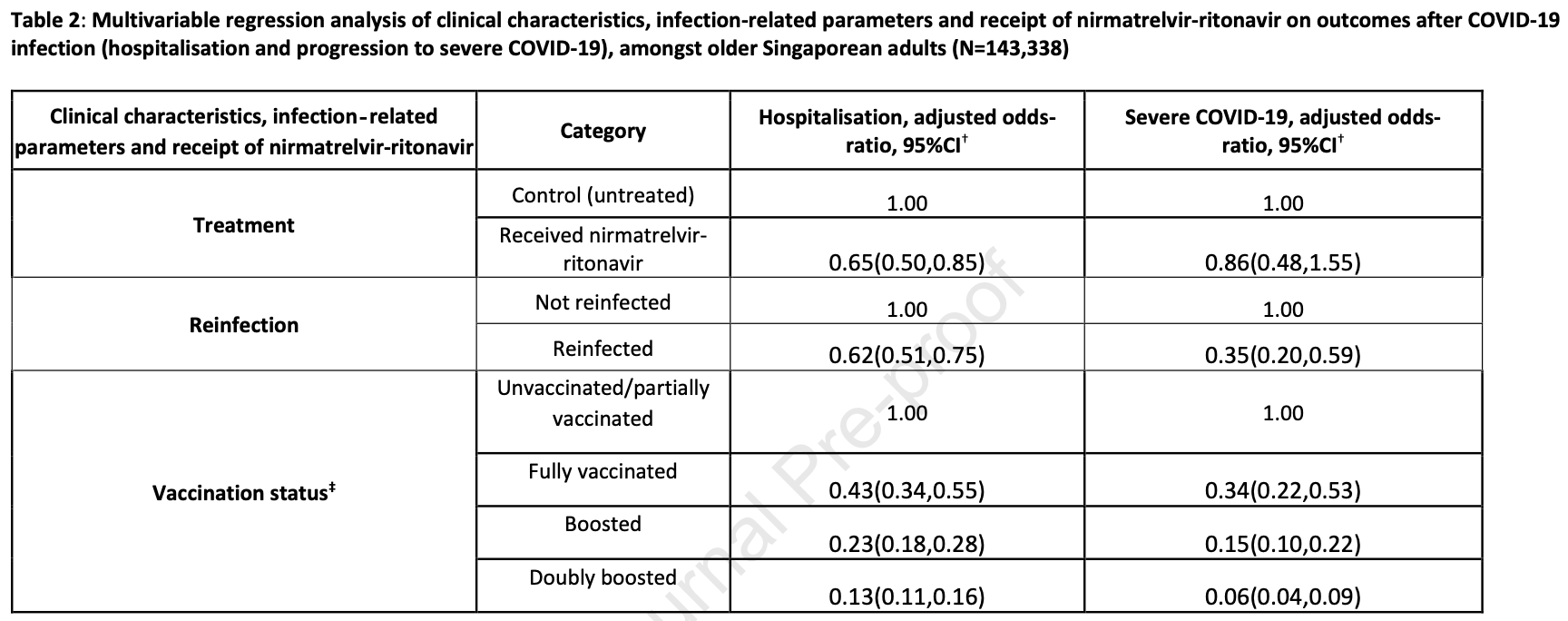
Real-world effectiveness of nirmatrelvir/ritonavir against COVID-19 hospitalisations and severe COVID-19 in community-dwelling elderly Singaporeans during Omicron BA.2, BA.4/5 and XBB transmission
et al., Clinical Microbiology and Infection, doi:10.1016/j.cmi.2023.06.016, Jun 2023
Retrospective 3,959 paxlovid patients and 139,379 untreated controls, showing lower hospitalization with treatment. Contraindicted patients were excluded.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending paxlovid also recommended them, or
because the patient seeking out paxlovid is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Malden et al. confirm significant bias in the use of paxlovid, showing that treated
patients are more likely to be from affluent neighborhoods, be more health-conscious, and
have better access to care. Campion et al. also show that female patients were more
likely to receive paxlovid, and studies show that female patients are significantly more
likely to be health-conscious, for example being more likely to take additional
non-prescription treatments.
Therefore, these kind of studies may
overestimate efficacy.
Resistance. Variants may be resistant to paxlovid6-13. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID14. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid15. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid16. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury17 and liver injury18,19. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound20-22.
|
risk of severe case, 14.0% lower, OR 0.86, p = 0.63, treatment 3,959, control 139,739, adjusted per study, multivariable, RR approximated with OR.
|
|
risk of hospitalization, 35.0% lower, OR 0.65, p = 0.002, treatment 3,959, control 139,739, adjusted per study, multivariable, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Malden et al., Predictors of nirmatrelvir–ritonavir receipt among COVID-19 patients in a large US health system, Scientific Reports, doi:10.1038/s41598-024-57633-7.
5.
Campion et al., Disparities in the Use of nirmatrelvir/ritonavir for COVID-19: A Retrospective Cohort Study, Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1809.
6.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
7.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
8.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
9.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
10.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
11.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
12.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
13.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
14.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
15.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
16.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
17.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
18.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
19.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
20.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
Wee et al., 17 Jun 2023, retrospective, Singapore, peer-reviewed, 11 authors, study period 18 March, 2022 - 31 December, 2022.
Contact: ian.wee.l.e@singhealth.com.sg.
Real-world effectiveness of nirmatrelvir/ritonavir against COVID-19 hospitalisations and severe COVID-19 in community-dwelling elderly Singaporeans during Omicron BA.2, BA.4/5 and XBB transmission
Clinical Microbiology and Infection, doi:10.1016/j.cmi.2023.06.016
Real-world effectiveness of nirmatrelvir/ritonavir against COVID-19 hospitalisations and severe COVID-19 in community-dwelling elderly Singaporeans during Omicron BA.2, BA.4/5 and XBB transmission
Declarations
Declaration of interests The authors report no conflicts of interest.
Contribution statement LEW contributed to literature search and writing of the manuscript. ATT, CC, BEY, BW, RL, CLL, JT, SV, DCL and KBT contributed to critical review and editing of the manuscript. DCL and KBT provided supervision. KBT, ATT contributed to study design, data collection, and data analysis. All authors had full access to all the data in the study and take responsibility for the decision to submit for publication. KBT and ATT directly accessed and verified the underlying data reported in the manuscript. J o u r n a l P r e -p r o o f
References
Aggarwal, Molina, Beaty, Bennett, Carlson et al., Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00056-7
Arbel, Sagy, Hoshen, Battat, Lavie et al., Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge, N Engl J Med, doi:10.1056/NEJMoa2204919
Bajema, Wang, Hynes, Rowneki, Hickok et al., Early Adoption of Anti-SARS-CoV-2 Pharmacotherapies Among US Veterans With Mild to Moderate COVID-19, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.41434
Britton, Embi, Levy, Effectiveness of COVID-19 mRNA Vaccines Against COVID-19-Associated Hospitalizations Among Immunocompromised Adults During SARS-CoV-2 Omicron Predominance -VISION Network, 10 States, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7142a4
Dryden-Peterson, Kim, Kim, Caniglia, Lennes et al., Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System : A Population-Based Cohort Study, Ann Intern Med, doi:10.7326/M22-2141
Genomics, Hope, Charlett, Chand, Ghani et al., Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study, Lancet, doi:10.1016/S0140-6736(22)00462-7
Gold, Kelleher, Magid, Jackson, Pennini et al., Dispensing of Oral Antiviral Drugs for Treatment of COVID-19 by Zip Code-Level Social Vulnerability -United States, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7125e1
Guenter, Who should receive oral antiviral therapy for severe acute respiratory syndrome coronavirus 2 infection in the omicron era? Choose wisely, Clin Microbiol Infect, doi:10.1016/j.cmi.2022.11.028
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle et al., EPIC-HR Investigators. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Imai, Ito, Kiso, Yamayoshi, Uraki et al., Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB, N Engl J Med, doi:10.1056/NEJMc2214302
Li, Thomas, Li, Addressing Extreme Propensity Scores via the Overlap Weights, Am J Epidemiol, doi:10.1093/aje/kwy201
Nyberg, Ferguson, Nash, Webster, Flaxman et al., None
Pfizer, additional-data-paxlovidtm-supporting Pfizer reports additional data on PAXLOVID supporting upcoming new drug application submission to U
Sackett, Deeks, Altman, Down with odds ratios! Evidence Based Medicine
Savinkina, Paltiel, Ross, Gonsalves, Population-Level Strategies for Nirmatrelvir/Ritonavir Prescribing-A Cost-effectiveness Analysis. Open Forum Infect Dis, doi:10.1093/ofid/ofac637
Schwartz, Wang, Tadrous, Langford, Daneman et al., Population-based evaluation of the effectiveness of nirmatrelvirritonavir for reducing hospital admissions and mortality from COVID-19, CMAJ, doi:10.1503/cmaj.221608
Tan, Chiew, Lee, Effectiveness of a Fourth Dose of COVID-19 mRNA Vaccine Against Omicron Variant Among Elderly People in Singapore, Ann Intern Med, doi:10.7326/M22-2042
Tan, Chiew, Pang, Vaccine effectiveness against Delta, Omicron BA.1, and BA.2 in a highly vaccinated Asian setting: a test-negative design study, Clin Microbiol Infect, doi:10.1016/j.cmi.2022.08.002
Tan, Lam, Matchar, Zee, Wong, Singapore's health-care system: key features, challenges, and shifts, Lancet, doi:10.1016/S0140-6736(21)00252-X
Wagstaff, Health systems in East Asia: what can developing countries learn from Japan and the Asian Tigers? Health Econ, doi:10.1002/hec.1180
Wai, Chan, Cheung, Wang, Chan et al., Association of Molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. Lancet Reg Health West Pac, doi:10.1016/j.lanwpc.2022.100602
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study, Lancet, doi:10.1016/S0140-6736(22)01586-0
Xie, Bowe, Al-Aly, Nirmatrelvir and risk of hospital admission or death in adults with covid-19: emulation of a randomized target trial using electronic health records, BMJ, doi:10.1136/bmj-2022-073312
Yip, Lui, Lai, Wong, Tse et al., Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients, Clin Infect Dis, doi:10.1093/cid/ciac687
DOI record:
{
"DOI": "10.1016/j.cmi.2023.06.016",
"ISSN": [
"1198-743X"
],
"URL": "http://dx.doi.org/10.1016/j.cmi.2023.06.016",
"alternative-id": [
"S1198743X2300294X"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Real-world effectiveness of nirmatrelvir/ritonavir against COVID-19 hospitalisations and severe COVID-19 in community-dwelling elderly Singaporeans during Omicron BA.2, BA.4/5 and XBB transmission"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Clinical Microbiology and Infection"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.cmi.2023.06.016"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6428-9999",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wee",
"given": "Liang En",
"sequence": "first"
},
{
"affiliation": [],
"family": "Tay",
"given": "An Ting",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chiew",
"given": "Calvin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Young",
"given": "Barnaby Edward",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wong",
"given": "Betty",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lim",
"given": "Ruth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Ching Li",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tan",
"given": "Joyce",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vasoo",
"given": "Shawn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lye",
"given": "David Chien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tan",
"given": "Kelvin Bryan",
"sequence": "additional"
}
],
"container-title": "Clinical Microbiology and Infection",
"container-title-short": "Clinical Microbiology and Infection",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalmicrobiologyandinfection.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
6,
17
]
],
"date-time": "2023-06-17T01:42:07Z",
"timestamp": 1686966127000
},
"deposited": {
"date-parts": [
[
2023,
6,
17
]
],
"date-time": "2023-06-17T01:42:26Z",
"timestamp": 1686966146000
},
"indexed": {
"date-parts": [
[
2023,
6,
17
]
],
"date-time": "2023-06-17T04:25:25Z",
"timestamp": 1686975925667
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1198743X2300294X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1198743X2300294X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
6
]
]
},
"published-print": {
"date-parts": [
[
2023,
6
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "10.1016/j.cmi.2023.06.016_bib1",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00462-7",
"article-title": "Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study",
"author": "Nyberg",
"doi-asserted-by": "crossref",
"first-page": "1303",
"issue": "10332",
"journal-title": "Lancet",
"key": "10.1016/j.cmi.2023.06.016_bib2",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2204919",
"article-title": "Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge",
"author": "Arbel",
"doi-asserted-by": "crossref",
"first-page": "790",
"issue": "9",
"journal-title": "N Engl J Med",
"key": "10.1016/j.cmi.2023.06.016_bib3",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.7326/M22-2141",
"article-title": "Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System : A Population-Based Cohort Study",
"author": "Dryden-Peterson",
"doi-asserted-by": "crossref",
"first-page": "77",
"issue": "1",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.cmi.2023.06.016_bib4",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(23)00011-7",
"article-title": "Real-world use of nirmatrelvir–ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study",
"author": "Aggarwal",
"doi-asserted-by": "crossref",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.cmi.2023.06.016_bib5",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(22)01586-0",
"author": "Wong",
"doi-asserted-by": "crossref",
"first-page": "1213",
"issue": "10359",
"journal-title": "Lancet",
"key": "10.1016/j.cmi.2023.06.016_bib6",
"volume": "400",
"year": "2022"
},
{
"article-title": "Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients",
"author": "Yip",
"first-page": "ciac687",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.cmi.2023.06.016_bib7",
"year": "2022"
},
{
"article-title": "Association of Molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19",
"author": "Wai",
"journal-title": "Lancet Reg Health West Pac",
"key": "10.1016/j.cmi.2023.06.016_bib8",
"volume": "30",
"year": "2023"
},
{
"key": "10.1016/j.cmi.2023.06.016_bib9",
"unstructured": "Pfizer, New York, USA. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-reports-additional-data-paxlovidtm-supporting Pfizer reports additional data on PAXLOVID supporting upcoming new drug application submission to U.S. FDA [press release]. 2022 [cited 2023 Jan 24]."
},
{
"DOI": "10.1503/cmaj.221608",
"article-title": "Population-based evaluation of the effectiveness of nirmatrelvir–ritonavir for reducing hospital admissions and mortality from COVID-19",
"author": "Schwartz",
"doi-asserted-by": "crossref",
"first-page": "E220",
"issue": "13",
"journal-title": "CMAJ",
"key": "10.1016/j.cmi.2023.06.016_bib10",
"volume": "195",
"year": "2023"
},
{
"article-title": "Nirmatrelvir and risk of hospital admission or death in adults with covid-19: emulation of a randomized target trial using electronic health records",
"author": "Xie",
"issue": "11",
"journal-title": "BMJ",
"key": "10.1016/j.cmi.2023.06.016_bib11",
"volume": "381",
"year": "2023"
},
{
"DOI": "10.1056/NEJMc2214302",
"article-title": "Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB",
"author": "Imai",
"doi-asserted-by": "crossref",
"first-page": "89",
"issue": "1",
"journal-title": "N Engl J Med",
"key": "10.1016/j.cmi.2023.06.016_bib12",
"volume": "388",
"year": "2023"
},
{
"key": "10.1016/j.cmi.2023.06.016_bib13",
"unstructured": "Ministry of Health, Singapore https://www.covid.gov.sg/well-and-positive-or-condition-assessed-mild-by-doctor/ COVID-19 protocols [Internet]. 2022 [cited 2023 Jan 24]"
},
{
"article-title": "Vaccine effectiveness against Delta, Omicron BA.1, and BA.2 in a highly vaccinated Asian setting: a test-negative design study",
"author": "Tan",
"first-page": "00418",
"issue": "22",
"journal-title": "Clin Microbiol Infect",
"key": "10.1016/j.cmi.2023.06.016_bib14",
"volume": "24",
"year": "2022"
},
{
"DOI": "10.7326/M22-2042",
"article-title": "Effectiveness of a Fourth Dose of COVID-19 mRNA Vaccine Against Omicron Variant Among Elderly People in Singapore",
"author": "Tan",
"doi-asserted-by": "crossref",
"first-page": "1622",
"issue": "11",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.cmi.2023.06.016_bib15",
"volume": "175",
"year": "2022"
},
{
"key": "10.1016/j.cmi.2023.06.016_bib16",
"unstructured": "Ministry of Health, Singapore https://www.moh.gov.sg/news-highlights/details/update-on-covid-19-situation-and-measures-to-protect-healthcare-capacity. Update on COVID-19 situation- XBB subvariant [Internet]. 2022 [cited 2023 Jan 24]"
},
{
"DOI": "10.1016/S0140-6736(21)00252-X",
"article-title": "Singapore's health-care system: key features, challenges, and shifts",
"author": "Tan",
"doi-asserted-by": "crossref",
"first-page": "1091",
"issue": "10305",
"journal-title": "Lancet",
"key": "10.1016/j.cmi.2023.06.016_bib17",
"volume": "398",
"year": "2021"
},
{
"DOI": "10.15585/mmwr.mm7142a4",
"article-title": "Effectiveness of COVID-19 mRNA Vaccines Against COVID-19-Associated Hospitalizations Among Immunocompromised Adults During SARS-CoV-2 Omicron Predominance - VISION Network, 10 States, December",
"author": "Britton",
"doi-asserted-by": "crossref",
"first-page": "1335",
"issue": "42",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "10.1016/j.cmi.2023.06.016_bib18",
"volume": "71",
"year": "2022"
},
{
"key": "10.1016/j.cmi.2023.06.016_bib19",
"unstructured": "Ministry of Health, Singapore https://www.moh.gov.sg/covid-19/vaccination/statistics Vaccination statistics [Internet]. 2022 [cited 2023 Jan 24]"
},
{
"key": "10.1016/j.cmi.2023.06.016_bib20",
"unstructured": "National Centre for Infectious Diseases, Singapore. Updated COVID-19 Therapy Guidance and Oral Antiviral Prescribing Checklist. [Internet]. 2022 [cited 2023 Jan 24]"
},
{
"DOI": "10.1002/hec.1180",
"article-title": "Health systems in East Asia: what can developing countries learn from Japan and the Asian Tigers?",
"author": "Wagstaff",
"doi-asserted-by": "crossref",
"first-page": "441",
"issue": "5",
"journal-title": "Health Econ",
"key": "10.1016/j.cmi.2023.06.016_bib21",
"volume": "16",
"year": "2007"
},
{
"key": "10.1016/j.cmi.2023.06.016_bib22",
"unstructured": "Centre for Evidence Based Medicine, Oxford, United Kingdom. Number needed to treat (NNT). [cited 2023 8th April]; Available from: http://www.cebm.net/number-needed-to-treat-nnt/. 2023."
},
{
"article-title": "Down with odds ratios",
"author": "Sackett",
"first-page": "164",
"issue": "6",
"journal-title": "Evidence Based Medicine",
"key": "10.1016/j.cmi.2023.06.016_bib23",
"volume": "1",
"year": "1996"
},
{
"article-title": "Addressing Extreme Propensity Scores via the Overlap Weights",
"author": "Li",
"first-page": "250",
"issue": "1",
"journal-title": "Am J Epidemiol",
"key": "10.1016/j.cmi.2023.06.016_bib24",
"volume": "188",
"year": "2019"
},
{
"article-title": "Who should receive oral antiviral therapy for severe acute respiratory syndrome coronavirus 2 infection in the omicron era? Choose wisely",
"author": "Guenter",
"first-page": "00601",
"issue": "22",
"journal-title": "Clin Microbiol Infect",
"key": "10.1016/j.cmi.2023.06.016_bib25",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1093/ofid/ofac637",
"article-title": "Population-Level Strategies for Nirmatrelvir/Ritonavir Prescribing-A Cost-effectiveness Analysis",
"author": "Savinkina",
"doi-asserted-by": "crossref",
"first-page": "ofac637",
"issue": "12",
"journal-title": "Open Forum Infect Dis",
"key": "10.1016/j.cmi.2023.06.016_bib26",
"volume": "9",
"year": "2022"
},
{
"key": "10.1016/j.cmi.2023.06.016_bib27",
"unstructured": "Ministry of Health, Singapore. https://www.moh.gov.sg/news-highlights/details/increased-accessibility-to-paxlovid-for-eligible-covid-19-patients Increased accessibility to paxlovid for eligible COVID-19 patients. [Internet]. 2022 [cited 2023 Jan 24]"
},
{
"DOI": "10.15585/mmwr.mm7125e1",
"article-title": "Dispensing of Oral Antiviral Drugs for Treatment of COVID-19 by Zip Code-Level Social Vulnerability - United States, December 23, 2021-May 21, 2022",
"author": "Gold",
"doi-asserted-by": "crossref",
"first-page": "825",
"issue": "25",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "10.1016/j.cmi.2023.06.016_bib28",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2022.41434",
"article-title": "Early Adoption of Anti-SARS-CoV-2 Pharmacotherapies Among US Veterans With Mild to Moderate COVID-19, January and February 2022",
"author": "Bajema",
"doi-asserted-by": "crossref",
"issue": "11",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.cmi.2023.06.016_bib29",
"volume": "5",
"year": "2022"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1198743X2300294X"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "Real-world effectiveness of nirmatrelvir/ritonavir against COVID-19 hospitalisations and severe COVID-19 in community-dwelling elderly Singaporeans during Omicron BA.2, BA.4/5 and XBB transmission",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}