Anti‑inflammatory effect of metformin against an experimental model of LPS‑induced cytokine storm
et al., Experimental and Therapeutic Medicine, doi:10.3892/etm.2023.12114, Jul 2023
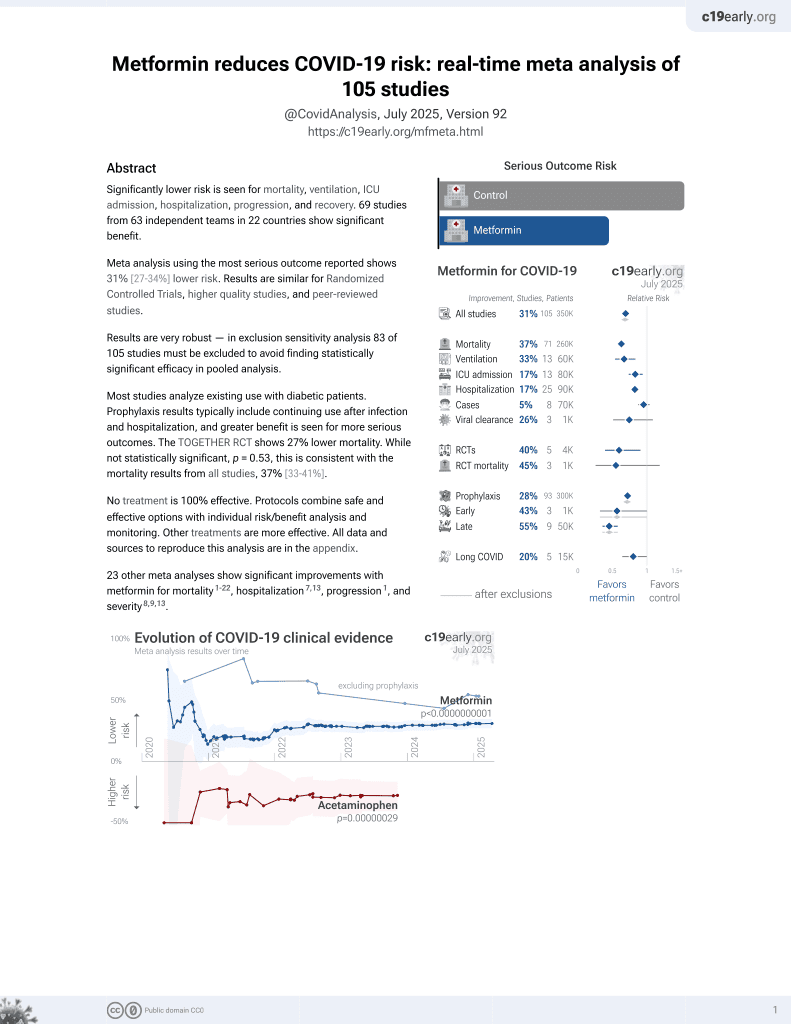
Metformin for COVID-19
3rd treatment shown to reduce risk in
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
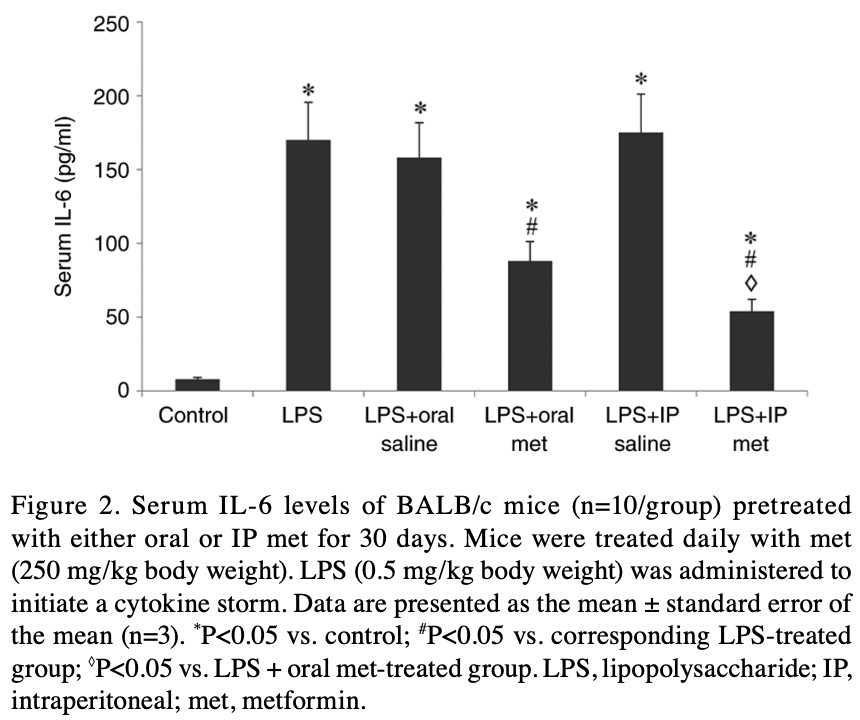
Mouse study showing that metformin attenuated LPS-induced increases in inflammatory cytokines, demonstrating an anti-inflammatory effect that may be useful against cytokine storm.
60 female mice were pretreated with oral or intraperitoneal (IP) metformin daily for 30 days. Lipopolysaccharide (LPS) was then injected to induce a cytokine storm and trigger inflammation. Metformin pretreatment, both oral and IP, significantly reduced the LPS-induced increase in serum levels of the proinflammatory cytokines IL-1β, IL-6, and TNF-α. IP metformin was more effective than oral metformin at reducing IL-6 levels. Neither oral nor IP metformin had a significant effect on IL-17 levels.
The results suggest metformin may have potential to suppress damaging inflammatory cytokine storms, suggesting benefit for treating cytokine storm-related diseases like COVID-19.
18 preclinical studies support the efficacy of metformin for COVID-19:
A systematic review and meta-analysis of 15 non-COVID-19 preclinical studies showed that metformin inhibits pulmonary inflammation and oxidative stress, minimizes lung injury, and improves survival in animal models of acute respiratory distress syndrome (ARDS) or acute lung injury (ALI)15.
Metformin inhibits SARS-CoV-2 in vitro11,12, minimizes LPS-induced cytokine storm in a mouse model14, minimizes lung damage and fibrosis in a mouse model of LPS-induced ARDS10, may protect against SARS-CoV-2-induced neurological disorders9, may be beneficial via inhibitory effects on ORF3a-mediated inflammasome activation16, reduces UUO and FAN-induced kidney fibrosis10, increases mitochondrial function and decreases TGF-β-induced fibrosis, apoptosis, and inflammation markers in lung epithelial cells10, may reduce inflammation, oxidative stress, and thrombosis via regulating glucose metabolism2, attenuates spike protein S1-induced inflammatory response and α-synuclein aggregation8, may protect against COVID-19 cognitive impairment by suppressing HIF-1α stabilization and reducing neurodegenerative protein aggregation13, may reduce COVID-19 severity and long COVID by inhibiting NETosis via suppression of protein kinase C activation17, enhances interferon responses and reduces SARS-CoV-2 infection and inflammation in diabetic models by suppressing HIF-1α signaling7, may improve COVID-19 outcomes by preventing VDAC1 mistargeting to the plasma membrane, reducing ATP loss, and preserving immune cell function during cytokine storm18, reduces hyperglycemia-induced hepatic ACE2/TMPRSS2 up-regulation and SARS-CoV-2 entry6, may reduce COVID-19 severity by suppressing monocyte inflammatory responses and glycolytic activation via AMPK pathway modulation5, and may improve outcomes via modulation of immune responses with increased anti-inflammatory T lymphocyte gene expression and via enhanced gut microbiota diversity19.
1.
Tavares et al., Investigation of Interactions Between the Protein MPro and the Vanadium Complex VO(metf)2∙H2O: A Computational Approach for COVID-19 Treatment, Biophysica, doi:10.3390/biophysica5010004.
2.
Hou et al., Metformin is a potential therapeutic for COVID-19/LUAD by regulating glucose metabolism, Scientific Reports, doi:10.1038/s41598-024-63081-0.
3.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
4.
Lockwood, T., Coordination chemistry suggests that independently observed benefits of metformin and Zn2+ against COVID-19 are not independent, BioMetals, doi:10.1007/s10534-024-00590-5.
5.
Maurmann et al., Immunoregulatory effect of metformin in monocytes exposed to SARS-CoV-2 spike protein subunit 1, bioRxiv, doi:10.1101/2025.09.12.675877.
6.
Rao et al., Pathological Glucose Levels Enhance Entry Factor Expression and Hepatic SARS‐CoV‐2 Infection, Journal of Cellular and Molecular Medicine, doi:10.1111/jcmm.70581.
7.
Joshi et al., Severe SARS‐CoV‐2 infection in diabetes was rescued in mice supplemented with metformin and/or αKG, and patients taking metformin, via HIF1α‐IFN axis, Clinical and Translational Medicine, doi:10.1002/ctm2.70275.
8.
Chang et al., SARS-CoV-2 Spike Protein 1 Causes Aggregation of α-Synuclein via Microglia-Induced Inflammation and Production of Mitochondrial ROS: Potential Therapeutic Applications of Metformin, Biomedicines, doi:10.3390/biomedicines12061223.
9.
Yang et al., SARS-CoV-2 infection causes dopaminergic neuron senescence, Cell Stem Cell, doi:10.1016/j.stem.2023.12.012.
10.
Miguel et al., Enhanced fatty acid oxidation through metformin and baicalin as therapy for COVID-19 and associated inflammatory states in lung and kidney, Redox Biology, doi:10.1016/j.redox.2023.102957.
11.
Ventura-López et al., Treatment with metformin glycinate reduces SARS-CoV-2 viral load: An in vitro model and randomized, double-blind, Phase IIb clinical trial, Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2022.113223.
12.
Parthasarathy et al., Metformin Suppresses SARS-CoV-2 in Cell Culture, bioRxiv, doi:10.1101/2021.11.18.469078.
13.
Lee et al., SARS-CoV-2 spike protein causes synaptic dysfunction and p-tau and α-synuclein aggregation leading cognitive impairment: The protective role of metformin, PLOS One, doi:10.1371/journal.pone.0336015.
14.
Taher et al., Anti‑inflammatory effect of metformin against an experimental model of LPS‑induced cytokine storm, Experimental and Therapeutic Medicine, doi:10.3892/etm.2023.12114.
15.
Wang et al., Effects of metformin on acute respiratory distress syndrome in preclinical studies: a systematic review and meta-analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2023.1215307.
16.
Zhang et al., SARS-CoV-2 ORF3a Protein as a Therapeutic Target against COVID-19 and Long-Term Post-Infection Effects, Pathogens, doi:10.3390/pathogens13010075.
17.
Monsalve et al., NETosis: A key player in autoimmunity, COVID-19, and long COVID, Journal of Translational Autoimmunity, doi:10.1016/j.jtauto.2025.100280.
Taher et al., 13 Jul 2023, peer-reviewed, 4 authors.
Contact: itaher@ju.edu.sa.
Anti‑inflammatory effect of metformin against an experimental model of LPS‑induced cytokine storm
Experimental and Therapeutic Medicine, doi:10.3892/etm.2023.12114
Cytokine storm is one of the leading causes of death in patients with COVID-19. Metformin has been shown to inhibit the action of a wide range of proinflammatory cytokines such as IL-6, and TNF-α which may ultimately affect cytokine storm due to Covid-19. The present study analyzed the anti-inflammatory effect of oral and intraperitoneal (IP) metformin administration routes in a mouse model of lipopolysaccharide (LPS)-induced cytokine storm. A total of 60 female BALB/c mice were randomly assigned to one of six groups: i) Control; ii) LPS model; iii) oral saline + LPS; iv) oral metformin + LPS; v) IP saline + LPS; and vi) IP metformin + LPS. Metformin or saline were administered to the mice for 30 days, after which an IP injection of 0.5 mg/kg LPS induced a cytokine storm in the five treatment groups. Mice were sacrificed and serum cytokine levels were measured. Pretreatment of mice with either oral or IP metformin significantly reduced the increase in IL-1, IL-6 and TNF-α following LPS injection. Both metformin administration routes significantly reduced IL-1 and TNF-α levels, although IP metformin appeared to be significantly more effective at reducing IL-6 levels compared with oral metformin. Neither the oral or IP route of administration of metformin demonstrated a significant effect on IL-17 levels. Based on its ability to suppress the proinflammatory LPS-induced cytokine storm, metformin may have future potential benefits in ameliorating human diseases caused by elevated cytokine levels.
Authors' contributions All of the authors have made substantial contributions towards the completion of the present study. MA and AET conceived the present study, performed the experiments, were project administrators and prepared the draft manuscript. IAT, EAEM, MA and AET collected the data, obtained resources, performed data analysis and critically reviewed and edited the manuscript. IAT and EAEM acquired funding. IAT, MA and AET supervised the project. IAT and AET confirm the authenticity of the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate Ethical approval was obtained from the Local Committee of Bioethics of Jouf University (approval no. 07-08-42; Sakaka, Saudi Arabia).
Patient consent for publication Not applicable.
Competing interests The authors declare that they have no competing interests.
References
Adeshirlarijaney, Zou, Tran, Chassaing, Gewirtz, Amelioration of metabolic syndrome by metformin associates with reduced indices of low-grade inflammation independently of the gut microbiota, Am J Physiol Endocrinol Metab
Ba, Xu, Yin, Yang, Wang et al., Metformin inhibits pro-inflammatory responses via targeting nuclear factor-κB in HaCaT cells, Cell Biochem Funct
Berliner, Hemophagocytic lymphohistiocytosis, Annu Rev Pathol
Carvalho, Aitken, Kumar, Gewirtz, Toll-like receptor-gut microbiota interactions: Perturb at your own risk!, Annu Rev Physiol
Catanzaro, Fagiani, Racchi, Corsini, Govoni, Immune response in COVID-19: Addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2, Signal Transduct Target Ther
Cavalli, Larcher, Tomelleri, Campochiaro, Della-Torre et al., Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID19 and hyperinfammation: A cohort study, Lancet Rheumatol
Channappanavar, Perlman, Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology, Semin Immunopathol
Chao, Hui-Jie, Fang, Ni, Kub, Anti-inflammatory effect of metformin LPS-induced inflammation in mice, Basic Clin Med
Cho, Song, Choi, Im, Hh et al., The suppressive effects of metformin on inflammatory response of otitis media model in human middle ear epithelial cells, Int J Pediatr Otorhinolaryngol
Chung, Nicol, Cheng, Lin, Chen et al., Metformin activation of AMPK suppresses AGE-induced inflammatory response in hNSCs, Exp Cell Res
Crayne, Albeituni, Nichols, Cron, The immunology of macrophage activation syndrome, Front Immunol
Dehkordi, Sattari, Khoshdel, Kasiri, Effect of folic acid and metformin on insulin resistance and inflammatory factors of obese children and adolescents, J Res Med Sci
Dias, Soares, Ferreira, Sacramento, Igues et al., Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators, PLoS Pathog
El A A, E S P I N O S A -Ol Iva A M, Sa Nt I A Go, García-Quintanilla, Oliva-Martín, Herrera et al., Metformin, besides exhibiting strong in vivo anti-inflammatory properties, increases mptp-induced damage to the nigrostriatal dopaminergic system, Toxicol Apple Pharmacol
Elbere, Kalnina, Silamikelis, Konrade, Zaharenko et al., Association of metformin administration with gut microbiome dysbiosis in healthy volunteers, PLoS One
Furuya, Kono, Hara, Hirayama, Sun et al., Interleukin 17A plays a role in lipopolysaccharide/D-galactosamine-induced fulminant hepatic injury in mice, J surg Res
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, Lancet
Hyun, Shin, Lee, Lee, Song et al., Metformin Down-regulates TNF-α secretion via suppression of scavenger receptors in macrophages, Immune Netw
Jang, Lee, Hong, Kwok, Cho et al., Metformin enhances the immunomodulatory potential of adipose-derived mesenchymal stem cells through STAT1 in an animal model of lupus, Rheumatology (Oxford)
Jing, Wu, Li, Yang, Li, Metformin improves obesity-associated inflammation by altering macrophages polarization, Mol Cell Endocrinol
Kelly, Tannahill, Murphy, Neill, Metformin inhibits the production of reactive oxygen species from NADH: Ubiquinone oxidoreductase to limit induction of interleukin-1β (IL-1β) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages, J Biol Chem
Kim, Kwak, Cha, Jeong, Rhee et al., Metformin suppresses lipopolysaccharide (LPS)-induced inflammatory response in murine macrophages via activating transcription factor-3 (ATF-3) induction, J Biol Chem
Kim, Lee, Lee, Kim, Jhun et al., Metformin ameliorates experimental-obesity-associated autoimmune arthritis by inducing FGF21 expression and brown adipocyte differentiation, Exp Mol Med
Kindler, Thiel, Weber, Interaction of SARS and MERS coronaviruses with the antiviral interferon response, Adv Virus Res
Koh, Kim, Kim, Ko, Kim, Anti-inflammatory mechanism of metformin and its effects in intestinal inflammation and colitis-associated colon cancer, J Gastroenterol Hepatol
Lee, Menezes, Umesaki, Mazmanian, Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis, Proc Natl Acad Sci
Lee, Moon, Kim, Seo, Yang et al., Metformin suppresses systemic autoimmunity in roquin san/san mice through inhibiting B cell differentiation into plasma cells via regulation of AMPK/mTOR/STAT3, J Immunol
Li, Geng, Peng, Meng, Lu, Molecular immune pathogenesis and diagnosis of COVID-19, J Pharm Anal
Li, Gu, Tu, Wang, Gu et al., Blockade of Interleukin-17 restrains the development of acute lung injury, Scand J Immunol
Liang, Wang, Chien, Yarmishyn, Yang et al., Highlight of immune pathogenic response and hematopathologic effect in SARS-CoV, MERS-CoV, and SARS-Cov-2 Infection
Liu, Liao, Zhang, Sun, Luo et al., Metformin affects gut microbiota composition and diversity associated with amelioration of dextran sulfate sodium-induced colitis in mice, Front Pharmacol
Lukens, Gurung, Vogel, Johnson, Carter et al., Dietary modulation of the microbiome affects autoinflammatory disease, Nature
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: Consider cytokine storm syndromes and immunosuppression, Lancet
Min, Cheon, Ha, Sohn, Kim et al., Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity, Sci Rep
Montazersaheb, Khatibi, Hejazi, Tarhriz, Farjami et al., COVID-19 infection: An overview on cytokine storm and related interventions, Virol J
Moon, Lee, Choi, Lee, Yoo et al., Metformin ameliorates scleroderma via inhibiting Th17 cells and reducing mTOR-STAT3 signaling in skin fibroblasts, J Transl Med
Park, Bang, Kwon, Moon, Kim et al., Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase, Biochem Pharmacol
Petrakis, Margină, Tsarouhas, Tekos, Stan et al., Obesity a risk factor for increased COVID19 prevalence, severity and lethality (Review), Mol Med Rep
Pfortmueller, Spinetti, Urman, Luedi, Schefold, COVID-19-associated acute respiratory distress syndrome (CARDS): Current knowledge on pathophysiology and ICU treatment-A narrative review, Best Pract Res Clin Anaesthesiol
Postler, Peng, Bhatt, Ghosh, Metformin selectively dampens the acute inflammatory response through an AMPK-dependent mechanism, Sci Rep
Rodriguez, Hiel, Delzenne, Metformin: Old friend, new ways of action-implication of the gut microbiome?, Curr Opin Clin Nutr Metab Care
Saisho, Metformin and inflammation: Its potential beyond glucose lowering effect, Endocr Metab Immune Disord Drug Targets
Sarzi-Puttini, Sirotti, Marotto, Ardizzone, Rizzardini et al., COVID-19, cytokines and immunosuppression: What can we learn from severe acute respiratory syndrome?, Clin Exp Rheumatol
Schuiveling, Vazirpanah, Radstake, Zimmermann, Broen, Metformin, a new era for an old drug in the treatment of immune mediated disease?, Curr Drug Targets
Sciannimanico, Grimaldi, Vescini, Pergola, Iacoviello et al., Metformin: Up to date, Endocr Metab Immune Disord Drug Targets
Sun, Zhang, Zhang, Zhang, Qian, IL-17A is implicated in lipopolysaccharide-induced neuroinflammation and cognitive impairment in aged rats via microglial activation, J Neuroinflammation
Tang, Yang, Chen, Shi, Ge et al., Metformin ameliorates sepsis-induced brain injury by inhibiting apoptosis, oxidative stress and neuroinflammation via the PI3K/Akt signaling pathway, Oncotarget
Yao, Zheng, Wu, Junhua, Immune environment modulation in pneumonia patients caused by coronavirus: SARS-CoV, MERS-CoV and SARS-CoV-2, Aging (Albany NY)
Yin, Choi, Xu, Perry, Seay et al., Normalization of CD4+ T cell metabolism reverses lupus, Sci Transl Med
Yuan, Jiao, Qu, Yang, Liu, The development of COVID-19 treatment, Front Immunol
Zhang, Hu, Effects of metformin on the gut microbiota in obesity and type 2 diabetes mellitus, Diabetes Metab Cinder Obes
Zhang, Xu, Chen, Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice, Biomed Pharmacother
Zhao, Cao, Liu, Li, Xu et al., Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression, Behav Brain Res
DOI record:
{
"DOI": "10.3892/etm.2023.12114",
"ISSN": [
"1792-0981",
"1792-1015"
],
"URL": "http://dx.doi.org/10.3892/etm.2023.12114",
"article-number": "415",
"author": [
{
"affiliation": [
{
"name": "Microbiology and Immunology Unit, Department of Pathology, College of Medicine, Jouf University, Sakaka 72388, Saudi Arabia"
}
],
"family": "Taher",
"given": "Ibrahim",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Microbiology and Immunology Unit, Department of Pathology, College of Medicine, Jouf University, Sakaka 72388, Saudi Arabia"
}
],
"family": "El‑Masry",
"given": "Eman",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacology and Therapeutics, College of Medicine, Jouf University, Sakaka 72388, Saudi Arabia"
}
],
"family": "Abouelkheir",
"given": "Mohamed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Microbiology and Immunology Unit, Department of Pathology, College of Medicine, Jouf University, Sakaka 72388, Saudi Arabia"
}
],
"family": "Taha",
"given": "Ahmed",
"sequence": "additional"
}
],
"container-title": "Experimental and Therapeutic Medicine",
"container-title-short": "Exp Ther Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
7,
13
]
],
"date-time": "2023-07-13T12:10:35Z",
"timestamp": 1689250235000
},
"deposited": {
"date-parts": [
[
2023,
7,
13
]
],
"date-time": "2023-07-13T12:10:36Z",
"timestamp": 1689250236000
},
"indexed": {
"date-parts": [
[
2023,
7,
14
]
],
"date-time": "2023-07-14T04:34:10Z",
"timestamp": 1689309250672
},
"is-referenced-by-count": 0,
"issue": "3",
"issued": {
"date-parts": [
[
2023,
7,
13
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2023,
7,
13
]
]
}
},
"member": "2249",
"original-title": [],
"prefix": "10.3892",
"published": {
"date-parts": [
[
2023,
7,
13
]
]
},
"published-online": {
"date-parts": [
[
2023,
7,
13
]
]
},
"publisher": "Spandidos Publications",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.spandidos-publications.com/10.3892/etm.2023.12114"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Cancer Research",
"Immunology and Microbiology (miscellaneous)",
"General Medicine"
],
"subtitle": [],
"title": "Anti‑inflammatory effect of metformin against an experimental model of LPS‑induced cytokine storm",
"type": "journal-article",
"volume": "26"
}