Metformin Alters mRNA Expression of FOXP3, RORC, and TBX21 and Modulates Gut Microbiota in COVID-19 Patients with Type 2 Diabetes
et al., Viruses, doi:10.3390/v16020281, Feb 2024
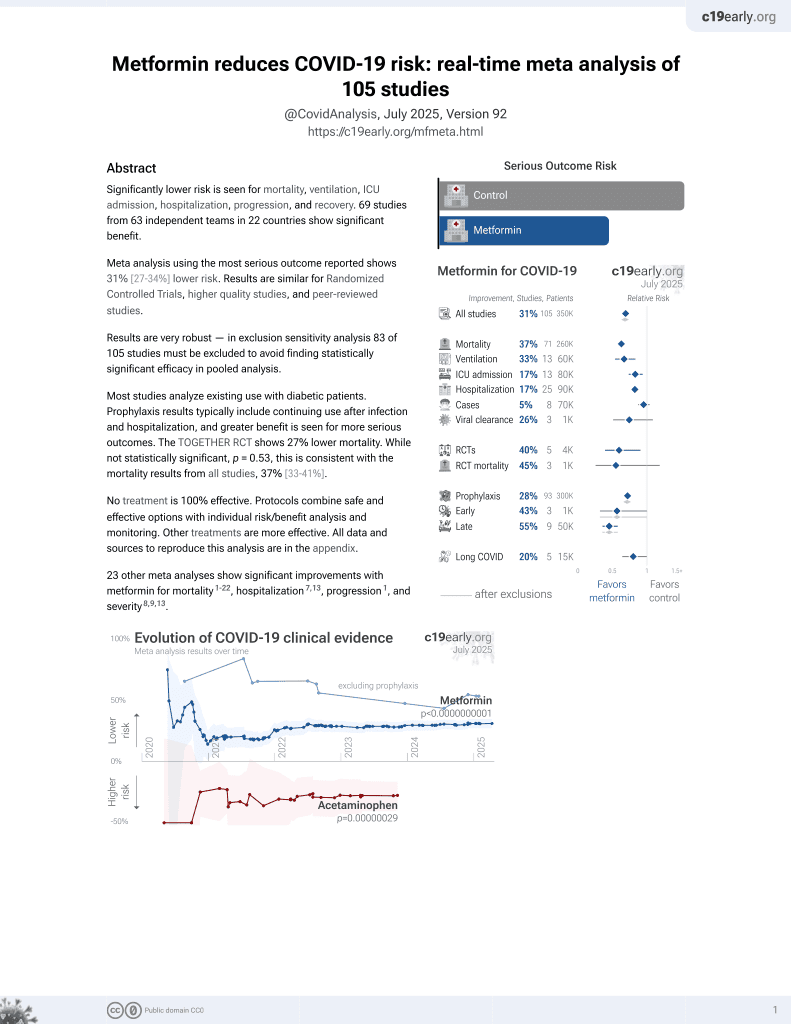
Metformin for COVID-19
3rd treatment shown to reduce risk in
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
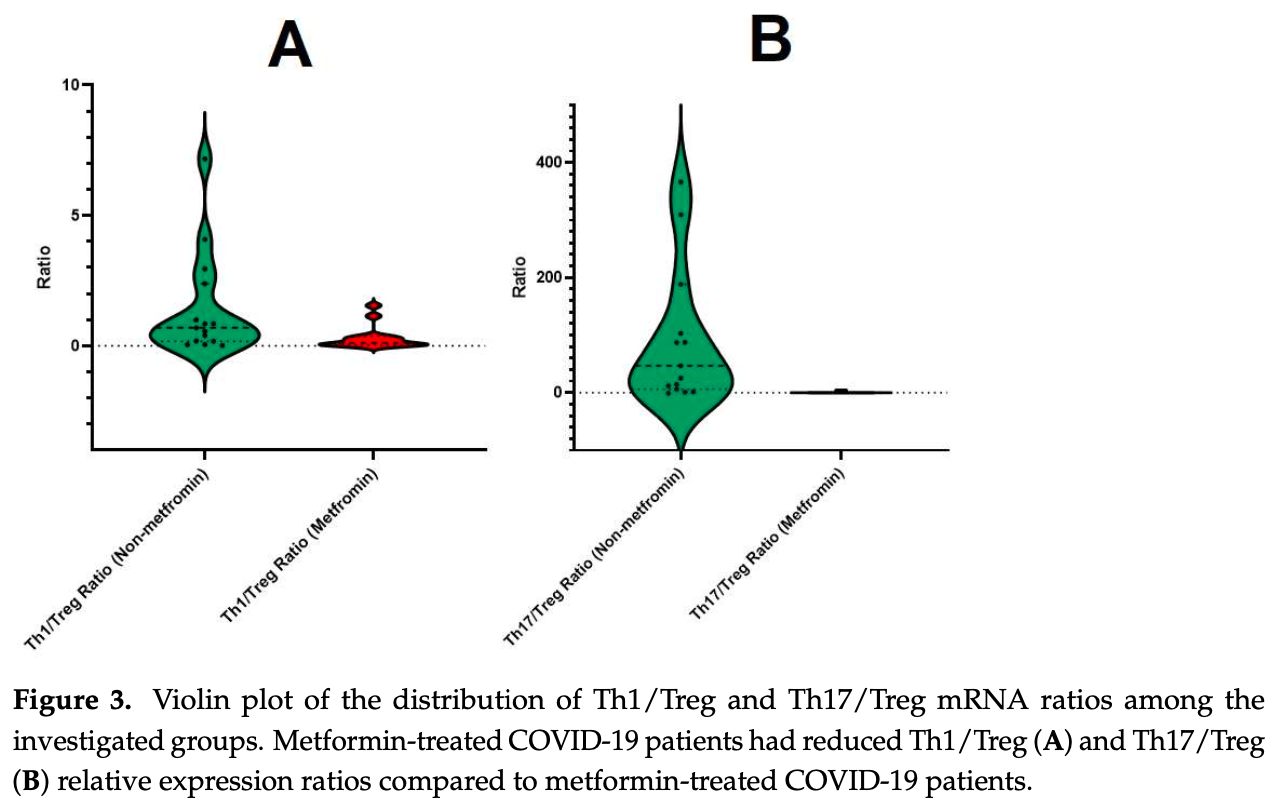
Retrospective 30 COVID-19 patients with type 2 diabetes showing improved T lymphocyte gene expression, gut microbiota diversity, and lower inflammatory markers with metformin treatment. Metformin-treated patients had a 1.96-fold increase in anti-inflammatory FOXP3 expression, and 1.84-fold and 11.4-fold decreases in pro-inflammatory RORC and TBX21 expression, respectively, compared to controls. Authors suggest that metformin treatment for type 2 diabetes patients with COVID-19 is promising based on modulation of immune responses and enhanced gut microbiota diversity.
Petakh et al., 11 Feb 2024, Ukraine, peer-reviewed, 4 authors.
Contact: pavlo.petakh@uzhnu.edu.ua (corresponding author), kamyshnyi_om@tdmu.edu.ua.
Metformin Alters mRNA Expression of FOXP3, RORC, and TBX21 and Modulates Gut Microbiota in COVID-19 Patients with Type 2 Diabetes
Viruses, doi:10.3390/v16020281
COVID-19 remains a significant global concern, particularly for individuals with type 2 diabetes who face an elevated risk of hospitalization and mortality. Metformin, a primary treatment for type 2 diabetes, demonstrates promising pleiotropic properties that may substantially mitigate disease severity and expedite recovery. Our study of the gut microbiota and the mRNA expression of pro-inflammatory and anti-inflammatory T-lymphocyte subpopulations showed that metformin increases bacterial diversity while modulating gene expression related to T-lymphocytes. This study found that people who did not take metformin had a downregulated expression of FOXP3 by 6.62fold, upregulated expression of RORC by 29.0-fold, and upregulated TBX21 by 1.78-fold, compared to the control group. On the other hand, metformin patients showed a 1.96-fold upregulation in FOXP3 expression compared to the control group, along with a 1.84-fold downregulation in RORC expression and an 11.4-fold downregulation in TBX21 expression. Additionally, we found a correlation with gut microbiota (F/B ratio and alpha-diversity index) and pro-inflammatory biomarkers. This novel observation of metformin's impact on T-cells and gut microbiota opens new horizons for further exploration through clinical trials to validate and confirm our data. The potential of metformin to modulate immune responses and enhance gut microbiota diversity suggests a promising avenue for therapeutic interventions in individuals with type 2 diabetes facing an increased risk of severe outcomes from COVID-19.
Author Contributions: Conceptualization, O.K.; methodology, O.K.; software, O.K.; validation, O.K. and P.P.; formal analysis, P.P.; investigation, O.K.; data curation, O.K.; writing-original draft preparation, P.P.; writing-review and editing, V.O.; visualization, P.P.; supervision, O.K.; project administration, I.K. All authors have read and agreed to the published version of the manuscript. Funding: This research received no external funding.
Institutional Review Board Statement: The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of I. Horbachevsky Ternopil National Medical University (protocol code 74 on 1 September 2023). Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest: The authors declare no conflicts of interest.
References
Bai, Chen, Metformin: A Novel Weapon Against Inflammation, Front. Pharmacol, doi:10.3389/fphar.2021.622262
Blagih, Coulombe, Vincent, Dupuy, Galicia-Vázquez et al., The Energy Sensor AMPK Regulates T Cell Metabolic Adaptation and Effector Responses In Vivo, Immunity, doi:10.1016/j.immuni.2014.12.030
Cao, Baranova, Wei, Wang, Zhang, Bidirectional causal associations between type 2 diabetes and COVID-19, J. Med. Virol, doi:10.1002/jmv.28100
Capone, Volpe, Transcriptional Regulators of T Helper 17 Cell Differentiation in Health and Autoimmune Diseases, Front. Immunol, doi:10.3389/fimmu.2020.00348
Chen, Zhou, Wang, Huang, Advances in metformin-based metabolic therapy for non-small cell lung cancer (Review), Oncol. Rep, doi:10.3892/or.2022.8266
Chi, Regulation and function of mTOR signalling in T cell fate decisions, Nat. Rev. Immunol, doi:10.1038/nri3198
De Gregoris, Aldred, Clare, Burgess, Improvement of phylum-and class-specific primers for real-time PCR quantification of bacterial taxa, J. Microbiol. Methods, doi:10.1016/j.mimet.2011.06.010
Hiscott, Alexandridi, Muscolini, Tassone, Palermo et al., The global impact of the coronavirus pandemic, Cytokine Growth Factor Rev, doi:10.1016/j.cytogfr.2020.05.010
Kamyshnyi, Matskevych, Lenchuk, Strilbytska, Storey et al., Metformin to decrease COVID-19 severity and mortality: Molecular mechanisms and therapeutic potential, Biomed. Pharmacother, doi:10.1016/j.biopha.2021.112230
Kondȇlková, Vokurková, Krejsek, Borská, Fiala et al., Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders, Acta Medica, doi:10.14712/18059694.2016.63
Livak, Schmittgen, Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2 -∆∆CT Method, Methods, doi:10.1006/meth.2001.1262
Looi, Scientists sound alarm over new BA.2.86 "Pirola" variant, BMJ Br. Med. J, doi:10.1136/bmj.p1964
Ma, Poffenberger, Wong, Jones, The role of AMPK in T cell metabolism and function, Curr. Opin. Immunol, doi:10.1016/j.coi.2017.04.004
Martonik, Parfieniuk-Kowerda, Rogalska, Flisiak, The Role of Th17 Response in COVID-19, Cells, doi:10.3390/cells10061550
Oestreich, Weinmann, Transcriptional mechanisms that regulate T helper 1 cell differentiation, Curr. Opin. Immunol, doi:10.1016/j.coi.2011.12.004
Petakh, Griga, Mohammed, Loshak, Poliak et al., Effects of Metformin, Insulin on Hematological Parameters of COVID-19 Patients with Type 2 Diabetes, Med. Arch, doi:10.5455/medarh.2022.76.329-332
Petakh, Isevych, Mohammed, Loshak, Poliak et al., Association between Use of Metformin and Insulin with Hematological Parameters in COVID-19 Patients with Type 2 Diabetes: A Single Center, Cross-Sectional Study, Clin. Diabetol, doi:10.5603/DK.a2022.0055
Petakh, Kamyshna, Kamyshnyi, Effects of metformin on the gut microbiota: A systematic review, Mol. Metab, doi:10.1016/j.molmet.2023.101805
Petakh, Kamyshna, Kamyshnyi, Gene expression of protein kinase AMP-activated catalytic subunit alpha 1 (PRKAA1), solute carrier family 2 member 1 (SLC2A1) and mechanistic target of rapamycin (MTOR) in metformin-treated type 2 diabetes patients with COVID-19: Impact on inflammation markers, Inflammopharmacology, doi:10.1007/s10787-023-01341-7
Petakh, Kamyshna, Kamyshnyi, Unveiling the potential pleiotropic effects of metformin in treating COVID-19: A comprehensive review, Front. Mol. Biosci, doi:10.3389/fmolb.2023.1260633
Petakh, Kamyshna, Nykyforuk, Yao, Imbery et al., Immunoregulatory Intestinal Microbiota and COVID-19 in Patients with Type Two Diabetes: A Double-Edged Sword, J. Viruses, doi:10.3390/v14030477
Petakh, Kamyshna, Oksenych, Kainov, Kamyshnyi, Metformin Therapy Changes Gut Microbiota Alpha-Diversity in COVID-19 Patients with Type 2 Diabetes: The Role of SARS-CoV-2 Variants and Antibiotic Treatment, Pharmaceuticals, doi:10.3390/ph16060904
Petakh, Kobyliak, Kamyshnyi, Gut microbiota in patients with COVID-19 and type 2 diabetes: A culture-based method, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2023.1142578
Petakh, Loshak, Kamyshnyi, Hematological features of patients with type 2 diabetes depending on the variant of SARS-CoV-2, Fiziolohichnyȋ Zhurnal, doi:10.15407/fz69.01.035
Petakh, Oksenych, Kamyshnyi, The F/B ratio as a biomarker for inflammation in COVID-19 and T2D: Impact of metformin, Biomed. Pharmacother, doi:10.1016/j.biopha.2023.114892
Polidori, Marullo, Ialongo, Tomassetti, Colombo et al., Characterization of Gut Microbiota Composition in Type 2 Diabetes Patients: A Population-Based Study, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph192315913
Reis, Silva, Silva, Thabane, Milagres et al., Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: The TOGETHER randomized platform clinical trial, Lancet Reg. Health Am
Rudensky, Regulatory T cells and Foxp3, Immunol. Rev, doi:10.1111/j.1600-065X.2011.01018.x
Salminen, Hyttinen, Kaarniranta, AMP-activated protein kinase inhibits NF-κB signaling and inflammation: Impact on healthspan and lifespan, J. Mol. Med, doi:10.1007/s00109-011-0748-0
Sharma, Behl, Sharma, Singh, Grewal et al., COVID-19 and diabetes: Association intensify risk factors for morbidity and mortality, Biomed. Pharmacother, doi:10.1016/j.biopha.2022.113089
Tulipano, Integrated or Independent Actions of Metformin in Target Tissues Underlying Its Current Use and New Possible Applications in the Endocrine and Metabolic Disorder Area, Int. J. Mol. Sci, doi:10.3390/ijms222313068
Ventura-López, Cervantes-Luevano, Aguirre-Sánchez, Flores-Caballero, Alvarez-Delgado et al., Treatment with metformin glycinate reduces SARS-CoV-2 viral load: An in vitro model and randomized, double-blind, Phase IIb clinical trial, Biomed. Pharmacother, doi:10.1016/j.biopha.2022.113223
Yu, Li, Sun, Wang, Insulin treatment is associated with increased mortality in patients with COVID-19 and type 2 diabetes, J. Cell Metab, doi:10.1016/j.cmet.2020.11.014
Zhao, Liu, Yi, Zhang, Xu et al., Type 2 diabetes mellitus impaired nasal immunity and increased the risk of hyposmia in COVID-19 mild pneumonia patients, Int. Immunopharmacol, doi:10.1016/j.intimp.2021.107406
Zhou, Myers, Li, Chen, Shen et al., Role of AMP-activated protein kinase in mechanism of metformin action, J. Clin. Investig, doi:10.1172/JCI13505
DOI record:
{
"DOI": "10.3390/v16020281",
"ISSN": [
"1999-4915"
],
"URL": "http://dx.doi.org/10.3390/v16020281",
"abstract": "<jats:p>COVID-19 remains a significant global concern, particularly for individuals with type 2 diabetes who face an elevated risk of hospitalization and mortality. Metformin, a primary treatment for type 2 diabetes, demonstrates promising pleiotropic properties that may substantially mitigate disease severity and expedite recovery. Our study of the gut microbiota and the mRNA expression of pro-inflammatory and anti-inflammatory T-lymphocyte subpopulations showed that metformin increases bacterial diversity while modulating gene expression related to T-lymphocytes. This study found that people who did not take metformin had a downregulated expression of FOXP3 by 6.62-fold, upregulated expression of RORC by 29.0-fold, and upregulated TBX21 by 1.78-fold, compared to the control group. On the other hand, metformin patients showed a 1.96-fold upregulation in FOXP3 expression compared to the control group, along with a 1.84-fold downregulation in RORC expression and an 11.4-fold downregulation in TBX21 expression. Additionally, we found a correlation with gut microbiota (F/B ratio and alpha-diversity index) and pro-inflammatory biomarkers. This novel observation of metformin’s impact on T-cells and gut microbiota opens new horizons for further exploration through clinical trials to validate and confirm our data. The potential of metformin to modulate immune responses and enhance gut microbiota diversity suggests a promising avenue for therapeutic interventions in individuals with type 2 diabetes facing an increased risk of severe outcomes from COVID-19.</jats:p>",
"alternative-id": [
"v16020281"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0860-4445",
"affiliation": [
{
"name": "Department of Biochemistry and Pharmacology, Uzhhorod National University, 88000 Uzhhorod, Ukraine"
},
{
"name": "Department of Microbiology, Virology, and Immunology, I. Horbachevsky Ternopil National Medical University, 46001 Ternopil, Ukraine"
}
],
"authenticated-orcid": false,
"family": "Petakh",
"given": "Pavlo",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-4483-1856",
"affiliation": [
{
"name": "Department of Medical Rehabilitation, I. Horbachevsky Ternopil National Medical University, 46001 Ternopil, Ukraine"
}
],
"authenticated-orcid": false,
"family": "Kamyshna",
"given": "Iryna",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5088-3791",
"affiliation": [
{
"name": "Broegelmann Research Laboratory, Department of Clinical Science, University of Bergen, 5020 Bergen, Norway"
}
],
"authenticated-orcid": false,
"family": "Oksenych",
"given": "Valentyn",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3141-4436",
"affiliation": [
{
"name": "Department of Microbiology, Virology, and Immunology, I. Horbachevsky Ternopil National Medical University, 46001 Ternopil, Ukraine"
}
],
"authenticated-orcid": false,
"family": "Kamyshnyi",
"given": "Oleksandr",
"sequence": "additional"
}
],
"container-title": "Viruses",
"container-title-short": "Viruses",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
2,
12
]
],
"date-time": "2024-02-12T09:47:45Z",
"timestamp": 1707731265000
},
"deposited": {
"date-parts": [
[
2024,
2,
12
]
],
"date-time": "2024-02-12T10:10:05Z",
"timestamp": 1707732605000
},
"indexed": {
"date-parts": [
[
2024,
2,
13
]
],
"date-time": "2024-02-13T00:25:37Z",
"timestamp": 1707783937618
},
"is-referenced-by-count": 0,
"issue": "2",
"issued": {
"date-parts": [
[
2024,
2,
11
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2024,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
11
]
],
"date-time": "2024-02-11T00:00:00Z",
"timestamp": 1707609600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4915/16/2/281/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "281",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2024,
2,
11
]
]
},
"published-online": {
"date-parts": [
[
2024,
2,
11
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1016/j.cytogfr.2020.05.010",
"article-title": "The global impact of the coronavirus pandemic",
"author": "Hiscott",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Cytokine Growth Factor Rev.",
"key": "ref_1",
"volume": "53",
"year": "2020"
},
{
"key": "ref_2",
"unstructured": "(2023, December 10). Available online: https://www.worldometers.info/coronavirus/."
},
{
"DOI": "10.1136/bmj.p1964",
"article-title": "COVID-19: Scientists sound alarm over new BA.2.86 “Pirola” variant",
"author": "Looi",
"doi-asserted-by": "crossref",
"first-page": "1964",
"journal-title": "BMJ Br. Med. J.",
"key": "ref_3",
"volume": "382",
"year": "2023"
},
{
"DOI": "10.1016/j.biopha.2022.113089",
"doi-asserted-by": "crossref",
"key": "ref_4",
"unstructured": "Sharma, P., Behl, T., Sharma, N., Singh, S., Grewal, A.S., Albarrati, A., Albratty, M., Meraya, A.M., and Bungau, S. (2022). COVID-19 and diabetes: Association intensify risk factors for morbidity and mortality. Biomed. Pharmacother., 151."
},
{
"DOI": "10.1002/jmv.28100",
"article-title": "Bidirectional causal associations between type 2 diabetes and COVID-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "e28100",
"journal-title": "J. Med. Virol.",
"key": "ref_5",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1016/j.cmet.2020.11.014",
"article-title": "Insulin treatment is associated with increased mortality in patients with COVID-19 and type 2 diabetes",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "65",
"journal-title": "J. Cell Metab.",
"key": "ref_6",
"volume": "33",
"year": "2021"
},
{
"DOI": "10.1016/j.intimp.2021.107406",
"article-title": "Type 2 diabetes mellitus impaired nasal immunity and increased the risk of hyposmia in COVID-19 mild pneumonia patients",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "107406",
"journal-title": "Int. Immunopharmacol.",
"key": "ref_7",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.3390/v14030477",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "Petakh, P., Kamyshna, I., Nykyforuk, A., Yao, R., Imbery, J.F., Oksenych, V., Korda, M., and Kamyshnyi, A. (2022). Immunoregulatory Intestinal Microbiota and COVID-19 in Patients with Type Two Diabetes: A Double-Edged Sword. J. Viruses, 14."
},
{
"DOI": "10.3389/fmolb.2023.1260633",
"doi-asserted-by": "crossref",
"key": "ref_9",
"unstructured": "Petakh, P., Kamyshna, I., and Kamyshnyi, A. (2023). Unveiling the potential pleiotropic effects of metformin in treating COVID-19: A comprehensive review. Front. Mol. Biosci., 10."
},
{
"DOI": "10.1016/j.biopha.2021.112230",
"doi-asserted-by": "crossref",
"key": "ref_10",
"unstructured": "Kamyshnyi, O., Matskevych, V., Lenchuk, T., Strilbytska, O., Storey, K., and Lushchak, O. (2021). Metformin to decrease COVID-19 severity and mortality: Molecular mechanisms and therapeutic potential. Biomed. Pharmacother., 144."
},
{
"DOI": "10.5455/medarh.2022.76.329-332",
"article-title": "Effects of Metformin, Insulin on Hematological Parameters of COVID-19 Patients with Type 2 Diabetes",
"author": "Petakh",
"doi-asserted-by": "crossref",
"first-page": "329",
"journal-title": "Med. Arch.",
"key": "ref_11",
"volume": "76",
"year": "2022"
},
{
"DOI": "10.15407/fz69.01.035",
"article-title": "Hematological features of patients with type 2 diabetes depending on the variant of SARS-CoV-2",
"author": "Petakh",
"doi-asserted-by": "crossref",
"first-page": "35",
"journal-title": "Fiziolohichnyĭ Zhurnal",
"key": "ref_12",
"volume": "69",
"year": "2023"
},
{
"DOI": "10.5603/DK.a2022.0055",
"article-title": "Association between Use of Metformin and Insulin with Hematological Parameters in COVID-19 Patients with Type 2 Diabetes: A Single Center, Cross-Sectional Study",
"author": "Petakh",
"doi-asserted-by": "crossref",
"first-page": "432",
"journal-title": "Clin. Diabetol.",
"key": "ref_13",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.3892/or.2022.8266",
"article-title": "Advances in metformin–based metabolic therapy for non–small cell lung cancer (Review)",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "55",
"journal-title": "Oncol. Rep.",
"key": "ref_14",
"volume": "47",
"year": "2022"
},
{
"DOI": "10.1038/nri3198",
"article-title": "Regulation and function of mTOR signalling in T cell fate decisions",
"author": "Chi",
"doi-asserted-by": "crossref",
"first-page": "325",
"journal-title": "Nat. Rev. Immunol.",
"key": "ref_15",
"volume": "12",
"year": "2012"
},
{
"DOI": "10.1016/j.immuni.2014.12.030",
"article-title": "The Energy Sensor AMPK Regulates T Cell Metabolic Adaptation and Effector Responses In Vivo",
"author": "Blagih",
"doi-asserted-by": "crossref",
"first-page": "41",
"journal-title": "Immunity",
"key": "ref_16",
"volume": "42",
"year": "2015"
},
{
"DOI": "10.3390/ijms222313068",
"doi-asserted-by": "crossref",
"key": "ref_17",
"unstructured": "Tulipano, G. (2021). Integrated or Independent Actions of Metformin in Target Tissues Underlying Its Current Use and New Possible Applications in the Endocrine and Metabolic Disorder Area. Int. J. Mol. Sci., 22."
},
{
"DOI": "10.1016/j.coi.2017.04.004",
"article-title": "The role of AMPK in T cell metabolism and function",
"author": "Ma",
"doi-asserted-by": "crossref",
"first-page": "45",
"journal-title": "Curr. Opin. Immunol.",
"key": "ref_18",
"volume": "46",
"year": "2017"
},
{
"DOI": "10.3389/fphar.2021.622262",
"article-title": "Metformin: A Novel Weapon Against Inflammation",
"author": "Bai",
"doi-asserted-by": "crossref",
"first-page": "622262",
"journal-title": "Front. Pharmacol.",
"key": "ref_19",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1111/j.1600-065X.2011.01018.x",
"article-title": "Regulatory T cells and Foxp3",
"author": "Rudensky",
"doi-asserted-by": "crossref",
"first-page": "260",
"journal-title": "Immunol. Rev.",
"key": "ref_20",
"volume": "241",
"year": "2011"
},
{
"DOI": "10.1016/j.coi.2011.12.004",
"article-title": "Transcriptional mechanisms that regulate T helper 1 cell differentiation",
"author": "Oestreich",
"doi-asserted-by": "crossref",
"first-page": "191",
"journal-title": "Curr. Opin. Immunol.",
"key": "ref_21",
"volume": "24",
"year": "2012"
},
{
"DOI": "10.3389/fimmu.2020.00348",
"article-title": "Transcriptional Regulators of T Helper 17 Cell Differentiation in Health and Autoimmune Diseases",
"author": "Capone",
"doi-asserted-by": "crossref",
"first-page": "348",
"journal-title": "Front. Immunol.",
"key": "ref_22",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3390/cells10061550",
"doi-asserted-by": "crossref",
"key": "ref_23",
"unstructured": "Martonik, D., Parfieniuk-Kowerda, A., Rogalska, M., and Flisiak, R. (2021). The Role of Th17 Response in COVID-19. Cells, 10."
},
{
"article-title": "Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders",
"author": "Krejsek",
"first-page": "73",
"journal-title": "Acta Medica",
"key": "ref_24",
"volume": "53",
"year": "2010"
},
{
"DOI": "10.3389/fcimb.2023.1142578",
"doi-asserted-by": "crossref",
"key": "ref_25",
"unstructured": "Petakh, P., Kobyliak, N., and Kamyshnyi, A. (2023). Gut microbiota in patients with COVID-19 and type 2 diabetes: A culture-based method. Front. Cell. Infect. Microbiol., 13."
},
{
"DOI": "10.1016/j.molmet.2023.101805",
"article-title": "Effects of metformin on the gut microbiota: A systematic review",
"author": "Petakh",
"doi-asserted-by": "crossref",
"first-page": "101805",
"journal-title": "Mol. Metab.",
"key": "ref_26",
"volume": "77",
"year": "2023"
},
{
"DOI": "10.1016/j.mimet.2011.06.010",
"article-title": "Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa",
"author": "Aldred",
"doi-asserted-by": "crossref",
"first-page": "351",
"journal-title": "J. Microbiol. Methods",
"key": "ref_27",
"volume": "86",
"year": "2011"
},
{
"key": "ref_28",
"unstructured": "(2016). Clinical Microbiology Procedures Handbook, ASM Press."
},
{
"DOI": "10.1006/meth.2001.1262",
"article-title": "Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method",
"author": "Livak",
"doi-asserted-by": "crossref",
"first-page": "402",
"journal-title": "Methods",
"key": "ref_29",
"volume": "25",
"year": "2001"
},
{
"DOI": "10.3390/ijerph192315913",
"doi-asserted-by": "crossref",
"key": "ref_30",
"unstructured": "Polidori, I., Marullo, L., Ialongo, C., Tomassetti, F., Colombo, R., di Gaudio, F., Calugi, G., Marrone, G., Noce, A., and Bernardini, S. (2022). Characterization of Gut Microbiota Composition in Type 2 Diabetes Patients: A Population-Based Study. Int. J. Environ. Res. Public Health, 19."
},
{
"DOI": "10.1016/j.biopha.2023.114892",
"doi-asserted-by": "crossref",
"key": "ref_31",
"unstructured": "Petakh, P., Oksenych, V., and Kamyshnyi, A. (2023). The F/B ratio as a biomarker for inflammation in COVID-19 and T2D: Impact of metformin. Biomed. Pharmacother., 163."
},
{
"DOI": "10.20944/preprints202304.1127.v1",
"doi-asserted-by": "crossref",
"key": "ref_32",
"unstructured": "Petakh, P., Kamyshna, I., Oksenych, V., Kainov, D., and Kamyshnyi, A. (2023). Metformin Therapy Changes Gut Microbiota Alpha-Diversity in COVID-19 Patients with Type 2 Diabetes: The Role of SARS-CoV-2 Variants and Antibiotic Treatment. Pharmaceuticals, 16."
},
{
"DOI": "10.1172/JCI13505",
"article-title": "Role of AMP-activated protein kinase in mechanism of metformin action",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1167",
"journal-title": "J. Clin. Investig.",
"key": "ref_33",
"volume": "108",
"year": "2001"
},
{
"DOI": "10.1007/s00109-011-0748-0",
"article-title": "AMP-activated protein kinase inhibits NF-κB signaling and inflammation: Impact on healthspan and lifespan",
"author": "Salminen",
"doi-asserted-by": "crossref",
"first-page": "667",
"journal-title": "J. Mol. Med.",
"key": "ref_34",
"volume": "89",
"year": "2011"
},
{
"DOI": "10.1007/s10787-023-01341-7",
"doi-asserted-by": "crossref",
"key": "ref_35",
"unstructured": "Petakh, P., Kamyshna, I., and Kamyshnyi, A. (2023). Gene expression of protein kinase AMP-activated catalytic subunit alpha 1 (PRKAA1), solute carrier family 2 member 1 (SLC2A1) and mechanistic target of rapamycin (MTOR) in metformin-treated type 2 diabetes patients with COVID-19: Impact on inflammation markers. Inflammopharmacology, 1–7."
},
{
"DOI": "10.1016/j.biopha.2022.113223",
"doi-asserted-by": "crossref",
"key": "ref_36",
"unstructured": "Ventura-López, C., Cervantes-Luevano, K., Aguirre-Sánchez, J.S., Flores-Caballero, J.C., Alvarez-Delgado, C., Bernaldez-Sarabia, J., Sánchez-Campos, N., Lugo-Sánchez, L.A., Rodríguez-Vázquez, I.C., and Sander-Padilla, J.G. (2022). Treatment with metformin glycinate reduces SARS-CoV-2 viral load: An in vitro model and randomized, double-blind, Phase IIb clinical trial. Biomed. Pharmacother., 152."
},
{
"article-title": "Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: The TOGETHER randomized platform clinical trial",
"author": "Reis",
"first-page": "100142",
"journal-title": "Lancet Reg. Health Am.",
"key": "ref_37",
"volume": "6",
"year": "2022"
}
],
"reference-count": 37,
"references-count": 37,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4915/16/2/281"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Infectious Diseases"
],
"subtitle": [],
"title": "Metformin Alters mRNA Expression of FOXP3, RORC, and TBX21 and Modulates Gut Microbiota in COVID-19 Patients with Type 2 Diabetes",
"type": "journal-article",
"volume": "16"
}