NETosis: A key player in autoimmunity, COVID-19, and long COVID
et al., Journal of Translational Autoimmunity, doi:10.1016/j.jtauto.2025.100280, Feb 2025
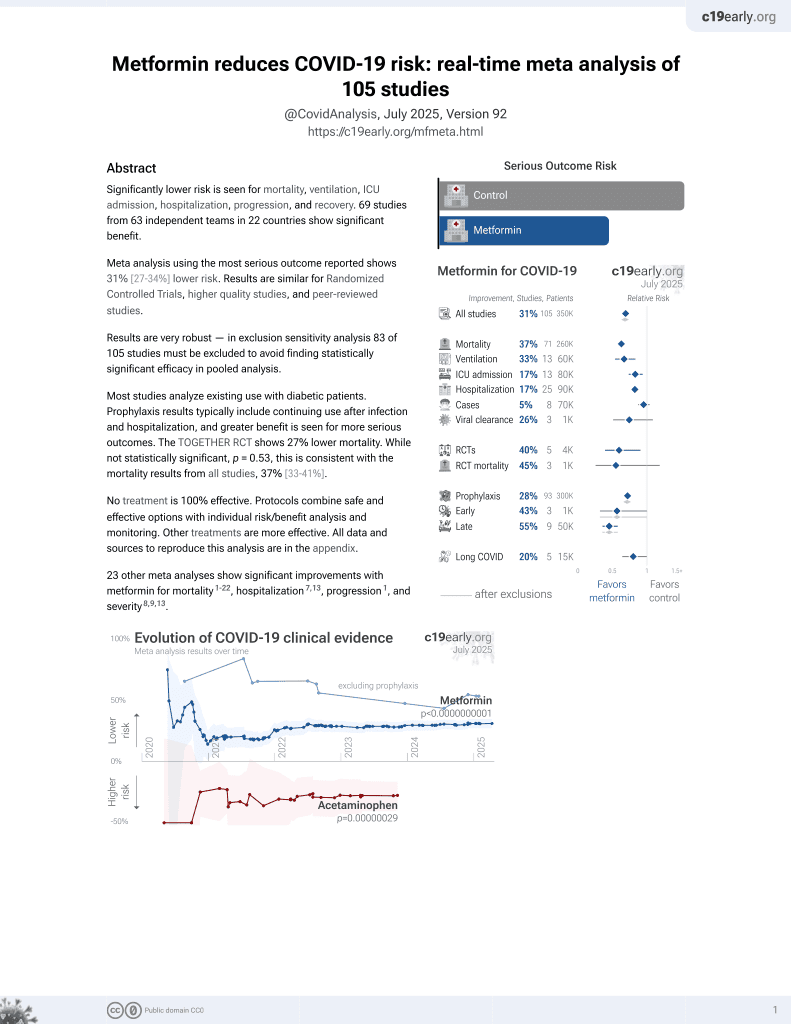
Metformin for COVID-19
3rd treatment shown to reduce risk in
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
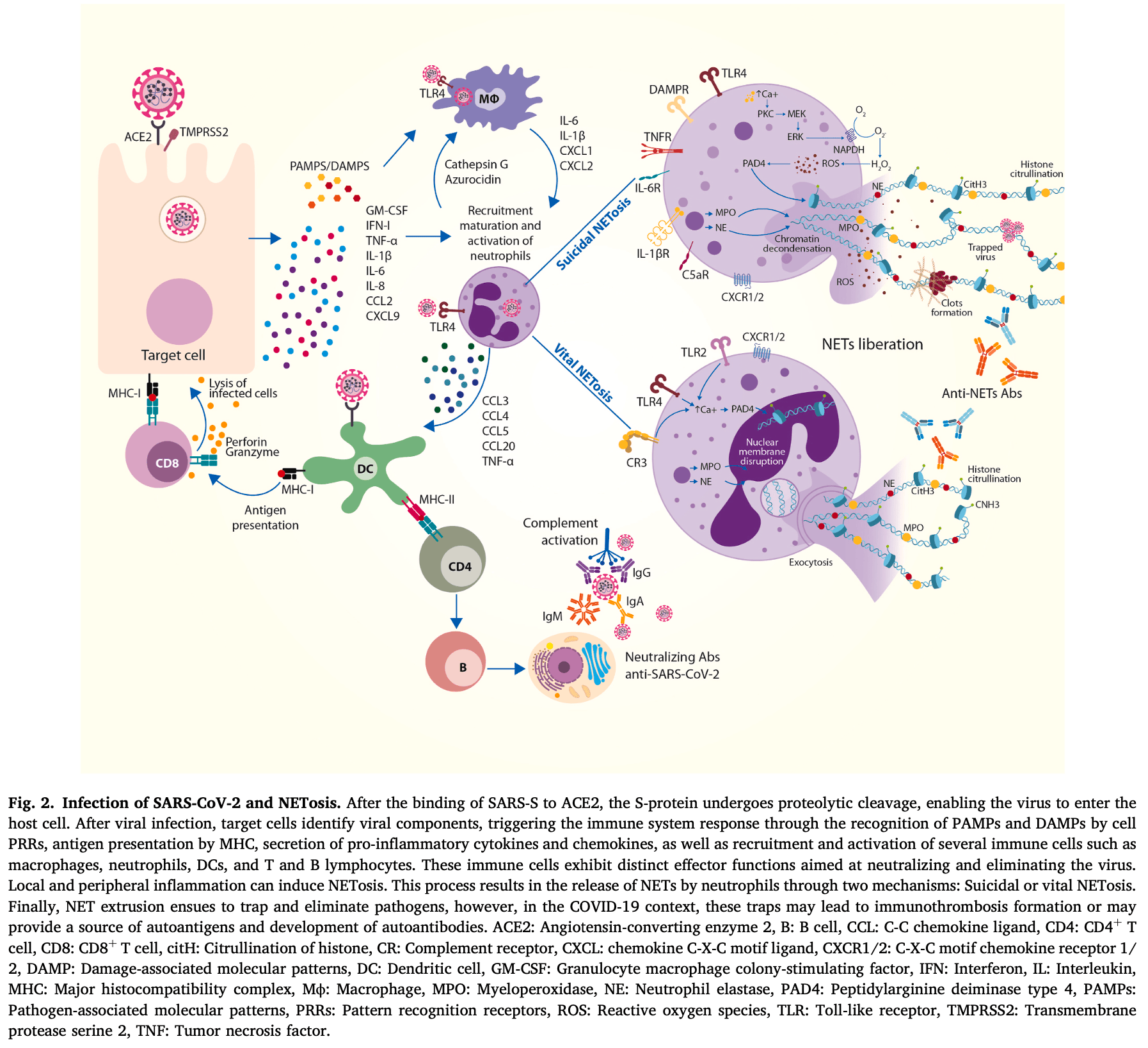
Review of neutrophil extracellular traps (NETs) and their role in autoimmunity, COVID-19, and long COVID. Authors explain that NETosis is a process where neutrophils release web-like structures (NETs) containing DNA, proteases, and enzymes to trap and kill pathogens. While crucial for host defense, dysregulated NET formation has been implicated in the pathogenesis of autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus, and antiphospholipid syndrome. During COVID-19, elevated neutrophil levels and excessive NET release contribute to coagulopathy, endothelial damage, respiratory failure, and immunothrombosis, correlating with disease severity and poor prognosis. Authors note that several therapeutics targeting NETs are potentially beneficial for COVID-19 or long COVID including metformin and hydroxychloroquine.
Review covers metformin and HCQ.
1.
Monsalve et al., NETosis: A key player in autoimmunity, COVID-19, and long COVID, Journal of Translational Autoimmunity, doi:10.1016/j.jtauto.2025.100280.
2.
Halabitska et al., Metformin in Antiviral Therapy: Evidence and Perspectives, Viruses, doi:10.3390/v16121938.
3.
Plowman et al., Anti-Inflammatory Potential of the Anti-Diabetic Drug Metformin in the Prevention of Inflammatory Complications and Infectious Diseases Including COVID-19: A Narrative Review, International Journal of Molecular Sciences, doi:10.3390/ijms25105190.
4.
De Jesús-González et al., A Dual Pharmacological Strategy against COVID-19: The Therapeutic Potential of Metformin and Atorvastatin, Microorganisms, doi:10.3390/microorganisms12020383.
5.
Halma et al., Exploring autophagy in treating SARS-CoV-2 spike protein-related pathology, Endocrine and Metabolic Science, doi:10.1016/j.endmts.2024.100163.
6.
Zhang et al., SARS-CoV-2 ORF3a Protein as a Therapeutic Target against COVID-19 and Long-Term Post-Infection Effects, Pathogens, doi:10.3390/pathogens13010075.
7.
Gomaa et al., Pharmacological evaluation of vitamin D in COVID-19 and long COVID-19: recent studies confirm clinical validation and highlight metformin to improve VDR sensitivity and efficacy, Inflammopharmacology, doi:10.1007/s10787-023-01383-x.
Monsalve et al., 21 Feb 2025, multiple countries, peer-reviewed, 8 authors.
Contact: heily.ramirez@urosario.edu.co.
NETosis: A key player in autoimmunity, COVID-19, and long COVID
Journal of Translational Autoimmunity, doi:10.1016/j.jtauto.2025.100280
NETosis, the process through which neutrophils release neutrophil extracellular traps (NETs), has emerged as a crucial mechanism in host defense and the pathogenesis of autoimmune responses. During the SARS-CoV-2 pandemic, this process received significant attention due to the central role of neutrophil recruitment and activation in infection control. However, elevated neutrophil levels and dysregulated NET formation have been linked to coagulopathy and endothelial damage, correlating with disease severity and poor prognosis in COVID-19. Moreover, it is known that SARS-CoV-2 can induce persistent low-grade systemic inflammation, known as long COVID, although the underlying causes remain unclear. It has been increasingly acknowledged that excessive NETosis and NET generation contribute to further pathophysiological abnormalities following SARS-CoV-2 infection. This review provides an updated overview of the role of NETosis in autoimmune diseases, but also the relationship between COVID-19 and long COVID with autoimmunity (e.g., latent and overt autoimmunity, molecular mimicry, epitope spreading) and NETosis (e.g., immune responses, NET markers). Finally, we discuss potential therapeutic strategies targeting dysregulated NETosis to mitigate the severe complications of COVID-19 and long COVID.
Abbreviations
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Ackermann, Anders, Bilyy, Bowlin, Daniel et al., Patients with COVID-19: in the dark-NETs of neutrophils, Cell Death Differ, doi:10.1038/s41418-021-00805-z
Acosta-Ampudia, Monsalve, Rojas, Rodríguez, Zapata et al., Persistent autoimmune activation and proinflammatory state in post-coronavirus disease 2019 syndrome, J. Infect. Dis, doi:10.1093/infdis/jiac017
Adiguzel, Molecular mimicry study between peptides of SARS-CoV-2 and neutrophil extracellular traps related proteins, doi:10.22541/au.170665898.84757134/v1
Adiguzel, Shoenfeld, Chapter 4 -Molecular Mimicry Study between Peptides of SARS-CoV-2 and Neutrophil Extracellular Traps-Related Proteins, doi:10.1016/B978-0-323-99130-8.00021-0
Al-Kuraishy, Al-Gareeb, Al-Hussaniy, Al-Harcan, Alexiou et al., Neutrophil Extracellular Traps (NETs) and Covid-19: a new frontiers for therapeutic modality, Int. Immunopharmacol, doi:10.1016/j.intimp.2021.108516
Alberti, Beretta, Piatti, Karantzoulis, Piatti et al., Guillain-Barré syndrome related to COVID-19 infection, Neurol. Neuroimmunol. Neuroinflammation, doi:10.1212/NXI.0000000000000741
Alkodaymi, Omrani, Fawzy, Shaar, Almamlouk et al., Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis, Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis, doi:10.1016/j.cmi.2022.01.014
Allez, Denis, Bouaziz, Battistella, Zagdanski et al., COVID-19-Related IgA vasculitis, Arthritis Rheumatol, doi:10.1002/art.41428
Ameer, Chaudhry, Mushtaq, Khan, Babar et al., An overview of systemic lupus erythematosus (SLE) pathogenesis, classification, and management, Cureus, doi:10.7759/cureus.30330
An, Eun, Yi, Park, CRESSP: a comprehensive pipeline for prediction of immunopathogenic SARS-CoV-2 epitopes using structural properties of proteins, Briefings Bioinf, doi:10.1093/bib/bbac056
Anaya, Monsalve, Rojas, Rodríguez, Montoya-García et al., Latent rheumatic, thyroid and phospholipid autoimmunity in hospitalized patients with COVID-19, J. Transl. Autoimmun, doi:10.1016/j.jtauto.2021.100091
Anaya, Rojas, Salinas, Rodríguez, Roa et al., Post-COVID syndrome. A case series and comprehensive review, Autoimmun. Rev, doi:10.1016/j.autrev.2021.102947
Angileri, Légaré, Marino, Gammazza, Conway De Macario et al., Is molecular mimicry the culprit in the autoimmune haemolytic anaemia affecting patients with COVID-19?, Br. J. Haematol, doi:10.1111/bjh.16883
Arango, Perricone, Kivity, Cipriano, Ceccarelli et al., HLA-DRB1 the notorious gene in the mosaic of autoimmunity, Immunol. Res, doi:10.1007/s12026-016-8817-7
Arneth, Arneth, Neutrophil extracellular traps (NETs) and vasculitis, Int. J. Med. Sci, doi:10.7150/ijms.53728
Aschenbrenner, Mouktaroudi, Krämer, Oestreich, Antonakos et al., Disease severity-specific neutrophil signatures in blood transcriptomes stratify COVID-19 patients, Genome Med, doi:10.1186/s13073-020-00823-5
Ashar, Mueller, Rudd, Snider, Achanta et al., The role of extracellular histones in influenza virus pathogenesis, Am. J. Pathol, doi:10.1016/j.ajpath.2017.09.014
Augustin, Schommers, Stecher, Dewald, Gieselmann et al., Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study, Lancet Reg. Heal. -Eur, doi:10.1016/j.lanepe.2021.100122
Aukrust, Holte, Opstad, Seljeflot, Berg et al., NETosis in long-term type 1 diabetes mellitus and its link to coronary artery disease, Front. Immunol, doi:10.3389/fimmu.2021.799539
Ayoubkhani, Khunti, Nafilyan, Maddox, Humberstone et al., Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study, BMJ, doi:10.1136/bmj.n693
Baj, Karakuła-Juchnowicz, Teresiński, Buszewicz, Ciesielka et al., COVID-19: specific and non-specific clinical manifestations and symptoms: the current state of knowledge, J. Clin. Med, doi:10.3390/jcm9061753
Bastard, Gervais, Voyer, Rosain, Philippot et al., None, doi:10.1126/sciimmunol.abl4340
Bertin, Kaphan, Weber, Babacci, Arcani et al., Persistent IgG anticardiolipin autoantibodies are associated with post-COVID syndrome, Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis, doi:10.1016/j.ijid.2021.09.079
Boeltz, Amini, Anders, Andrade, Bilyy et al., To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps, Cell Death Differ, doi:10.1038/s41418-018-0261-x
Bramante, Buse, Liebovitz, Nicklas, Puskarich et al., Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadrupleblind, parallel-group, phase 3 trial, Lancet Infect. Dis, doi:10.1016/S1473-3099(23)00299-2
Branzk, Papayannopoulos, Molecular mechanisms regulating NETosis in infection and disease, Semin. Immunopathol, doi:10.1007/s00281-013-0384-6
Bull-Otterson, Baca, Saydah, Boehmer, Adjei et al., Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥65 Years -United States, march 2020-november 2021, Morb. Mortal. Wkly. Rep, doi:10.15585/mmwr.mm7121e1
Burn, Foti, Marsman, Patel, Zychlinsky, The neutrophil, Immunity, doi:10.1016/j.immuni.2021.06.006
Busnadiego, Abela, Frey, Hofmaenner, Scheier et al., Critically ill COVID-19 patients with neutralizing autoantibodies against type I interferons have increased risk of herpesvirus disease, PLoS Biol, doi:10.1371/journal.pbio.3001709
Cabral-Marques, Halpert, Schimke, Ostrinski, Vojdani et al., Autoantibodies targeting GPCRs and RAS-related molecules associate with COVID-19 severity, Nat. Commun, doi:10.1038/s41467-022-28905-5
Castanheira, Kubes, Neutrophils and NETs in modulating acute and chronic inflammation, Blood, doi:10.1182/blood-2018-11-844530
Castanheira, Kubes, Neutrophils during SARS-CoV-2 infection: friend or foe?, Immunol. Rev, doi:10.1111/imr.13175
Cavazzana, Vojinovic, Airo, Fredi, Ceribelli et al., Systemic sclerosis-specific antibodies: novel and classical biomarkers, Clin. Rev. Allergy Immunol, doi:10.1007/s12016-022-08946-w
Chang, Feng, Meng, Apostolidis, Mack et al., New-onset IgG autoantibodies in hospitalized patients with COVID-19, Nat. Commun, doi:10.1038/s41467-021-25509-3
Chang, Yen-Ting Chen, Wang, Hung, Chen et al., Risk of autoimmune diseases in patients with COVID-19: a retrospective cohort study, EClinicalMedicine, doi:10.1016/j.eclinm.2022.101783
Chen, Zhang, Hu, Cai, Ni et al., Role of NETosis in central nervous system injury, Oxid. Med. Cell. Longev, doi:10.1155/2022/3235524
Chowdhury, Giaglis, Walker, Buser, Hahn et al., Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility, Arthritis Res. Ther, doi:10.1186/ar4579
Cui, Tan, Fan, Immunopathological roles of neutrophils in virus infection and COVID-19, Shock, doi:10.1097/SHK.0000000000001740
Davis, Assaf, Mccorkell, Wei, Low et al., Characterizing long COVID in an international cohort: 7 months of symptoms and their impact, EClinicalMedicine, doi:10.1016/j.eclinm.2021.101019
Davis, Mccorkell, Vogel, Topol, Long COVID: major findings, mechanisms and recommendations, Nat. Rev. Microbiol, doi:10.1038/s41579-022-00846-2
De Bont, Boelens, Pruijn, NETosis, complement, and coagulation: a triangular relationship, Cell. Mol. Immunol, doi:10.1038/s41423-018-0024-0
De Diego, Lasierra, López-Vergara, Torralba, Ruiz De Gopegui et al., What is the actual relationship between neutrophil extracellular traps and COVID-19 severity? A longitudinal study, Respir. Res, doi:10.1186/s12931-023-02650-9
De Moraes Mazetto, Hounkpe, Da Silva Saraiva, Vieira-Damiani, Santos et al., Association between neutrophil extracellular traps (NETs) and thrombosis in antiphospholipid syndrome, Thromb. Res, doi:10.1016/j.thromres.2022.05.001
Deane, Holers, Rheumatoid arthritis pathogenesis, prediction, and prevention: an emerging paradigm shift, Arthritis Rheumatol, doi:10.1002/art.41417
Del Giudice, Boudoumi, Le Guen, Reverte, Gutnecht et al., Catastrophic acute bilateral lower limbs necrosis associated with COVID-19 as a likely consequence of both vasculitis and coagulopathy, J. Eur. Acad. Dermatol. Venereol, doi:10.1111/jdv.16763
Delorey, Ziegler, Heimberg, Normand, Yang et al., None, doi:10.1038/s41586-021-03570-8
Didier, Giusti, Le Jan, Terryn, Muller et al., Neutrophil extracellular traps generation relates with early stage and vascular complications in systemic sclerosis, J. Clin. Med, doi:10.3390/jcm9072136
Dimeglio, Evans-Molina, Oram, Type 1 diabetes, Lancet, doi:10.1016/S0140-6736(18)31320-5
Domeier, Chodisetti, Schell, Kawasawa, Fasnacht et al., B-Cell-Intrinsic type 1 interferon signaling is crucial for loss of tolerance and the development of autoreactive B cells, Cell Rep, doi:10.1016/j.celrep.2018.06.046
Dotan, Kanduc, Muller, Makatsariya, Shoenfeld, Molecular mimicry between SARS-CoV-2 and the female reproductive system, Am. J. Reprod. Immunol, doi:10.1111/aji.13494
Dotan, Muller, Kanduc, David, Halpert et al., The SARS-CoV-2 as an instrumental trigger of autoimmunity, Autoimmun. Rev, doi:10.1016/j.autrev.2021.102792
Dotan, Shoenfeld, Post-COVID syndrome: the aftershock of SARS-CoV-2, Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis, doi:10.1016/j.ijid.2021.11.020
Ehrenfeld, Tincani, Andreoli, Cattalini, Greenbaum et al., Covid-19 and autoimmunity, Autoimmun. Rev, doi:10.1016/j.autrev.2020.102597
Elliott, Guda, Asuthkar, Teluguakula, Prasad et al., PAD inhibitors as a potential treatment for SARS-CoV-2 immunothrombosis, Biomedicines, doi:10.3390/biomedicines9121867
Esendagli, Yilmaz, Akçay, Özlü, Post-COVID syndrome: pulmonary complications, Turk. J. Med. Sci, doi:10.3906/sag-2106-238
Falk, Terrell, Charles, Jennette, Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro, Proc. Natl. Acad. Sci, doi:10.1073/pnas.87.11.4115
Gagiannis, Steinestel, Hackenbroch, Schreiner, Hannemann et al., Clinical, serological, and histopathological similarities between severe COVID-19 and acute exacerbation of connective tissue disease-associated interstitial lung disease (CTD-ILD), Front. Immunol, doi:10.3389/fimmu.2020.587517
Garcia-Romo, Caielli, Vega, Connolly, Allantaz et al., Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus, Sci. Transl. Med, doi:10.1126/scitranslmed.3001201
George, Reed, Desai, Devaraj, Faiez et al., A persistent neutrophil-associated immune signature characterizes post-COVID-19 pulmonary sequelae, Sci. Transl. Med, doi:10.1126/scitranslmed.abo5795
Guan, Ni, Hu, Liang, Ou et al., Clinical characteristics of coronavirus disease 2019 in China, N. Engl. J. Med, doi:10.1056/NEJMoa2002032
Guedes De Sa, Silva, Bayarri-Olmos, Brinda, Alec Rath Constable et al., A causal link between autoantibodies and neurological symptoms in long COVID, MedRxiv Prepr, Serv. Heal. Sci, doi:10.1101/2024.06.18.24309100
Gupta, Nakabo, Blanco, O'neil, Wigerblad et al., Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism, Proc. Natl. Acad. Sci. U.S.A, doi:10.1073/pnas.2003603117
Haffke, Freitag, Rudolf, Seifert, Doehner et al., Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS), J. Transl. Med, doi:10.1186/s12967-022-03346-2
Hakkim, Fuchs, Martinez, Hess, Prinz et al., Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation, Nat. Chem. Biol, doi:10.1038/nchembio.496
Hakkim, Fürnrohr, Amann, Laube, Abed et al., Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis, Proc. Natl. Acad. Sci, doi:10.1073/pnas.0909927107
Halpert, Shoenfeld, SARS-CoV-2, the autoimmune virus, Autoimmun. Rev, doi:10.1016/j.autrev.2020.102695
Hanson, Liu-Fei, Ng, Minato, Lai et al., Characterization of COVID-19-associated cardiac injury: evidence for a multifactorial disease in an autopsy cohort, Lab. Invest, doi:10.1038/s41374-022-00783-x
Hazeldine, Lord, Neutrophils and COVID-19: active participants and rational therapeutic targets, Front. Immunol, doi:10.3389/fimmu.2021.680134
Herrera, Bosch, Lok, Nguyen, Lenae et al., Circulating neutrophil extracellular trap (NET)-forming "rogue" neutrophil subset, immunotype [DEspR +CD11b+], mediate multi-organ failure in COVID-19 -an observational study, Res. Sq, doi:10.21203/rs.3.rs-2479844/v1
Hocini, Wiedemann, Blengio, Lefebvre, Cervantes-Gonzalez et al., Neutrophil activation and immune thrombosis profiles persist in convalescent COVID-19, J. Clin. Immunol, doi:10.1007/s10875-023-01459-x
Hu, Guo, Zhou, Shi, Characteristics of SARS-CoV-2 and COVID-19, Nat. Rev. Microbiol, doi:10.1038/s41579-020-00459-7
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Huckriede, Anderberg, Morales, Vries, Hultström et al., Evolution of NETosis markers and DAMPs have prognostic value in critically ill COVID-19 patients, Sci. Rep, doi:10.1038/s41598-021-95209-x
Inokuchi, Shimamoto, Persistent risk of developing autoimmune diseases associated with COVID-19: an observational study using an electronic medical record database in Japan, J. Clin. Rheumatol. Pract. Reports Rheum. Musculoskelet. Dis, doi:10.1097/RHU.0000000000002054
Jackson, Farzan, Chen, Choe, Mechanisms of SARS-CoV-2 entry into cells, Nat. Rev. Mol. Cell Biol, doi:10.1038/s41580-021-00418-x
Jamal, Bangash, Habiba, Lei, Xie et al., Immune dysregulation and system pathology in COVID-19, Virulence, doi:10.1080/21505594.2021.1898790
Jorch, Kubes, An emerging role for neutrophil extracellular traps in noninfectious disease, Nat. Med, doi:10.1038/nm.4294
Kanduc, Shoenfeld, Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine, Immunol. Res, doi:10.1007/s12026-020-09152-6
Kessenbrock, Krumbholz, Schönermarck, Back, Gross et al., Netting neutrophils in autoimmune smallvessel vasculitis, Nat. Med, doi:10.1038/nm.1959
Khandpur, Carmona-Rivera, Vivekanandan-Giri, Gizinski, Yalavarthi et al., NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis, Sci. Transl. Med, doi:10.1126/scitranslmed.3005580
Kiefer, Oropallo, Cancro, Marshak-Rothstein, Role of type I interferons in the activation of autoreactive B cells, Immunol. Cell Biol, doi:10.1038/icb.2012.10
Kitching, Anders, Basu, Brouwer, Gordon et al., ANCAassociated vasculitis, Nat. Rev. Dis. Primers, doi:10.1038/s41572-020-0204-y
Klok, Kruip, Van Der Meer, Arbous, Gommers et al., Incidence of thrombotic complications in critically ill ICU patients with COVID-19, Thromb. Res, doi:10.1016/j.thromres.2020.04.013
Knight, Kanthi, Mechanisms of immunothrombosis and vasculopathy in antiphospholipid syndrome, Semin. Immunopathol, doi:10.1007/s00281-022-00916-w
Kow, Ramachandram, Hasan, Metformin therapy in COVID-19: inhibition of NETosis, J. Thromb. Thrombolysis, doi:10.1007/s11239-022-02667-9
Krinsky, Sizikov, Nissim, Dror, Sas et al., NETosis induction reflects COVID-19 severity and long COVID: insights from a 2-center patient cohort study in Israel, J. Thromb. Haemostasis, doi:10.1016/j.jtha.2023.02.033
Kuley, Stultz, Duvvuri, Wang, Fritzler et al., N-formyl methionine peptide-mediated neutrophil activation in systemic sclerosis, Front. Immunol, doi:10.3389/fimmu.2021.785275
Lande, Ganguly, Facchinetti, Frasca, Conrad et al., Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus, Sci. Transl. Med, doi:10.1126/scitranslmed.3001180
Lazarian, Quinquenel, Bellal, Siavellis, Jacquy et al., Autoimmune haemolytic anaemia associated with COVID-19 infection, Br. J. Haematol, doi:10.1111/bjh.16794
Leffler, Martin, Gullstrand, Tydén, Lood et al., Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease, J. Immunol, doi:10.4049/jimmunol.1102404
Leng, Shah, Ahmad, Premraj, Wildi et al., Pathogenesis underlying neurological manifestations of long COVID syndrome and potential therapeutics, Cells, doi:10.3390/cells12050816
Leppkes, Knopf, Naschberger, Lindemann, Singh et al., Vascular occlusion by neutrophil extracellular traps in COVID-19, EBioMedicine, doi:10.1016/j.ebiom.2020.102925
Lewis, Liddle, Coote, Atkinson, Barker et al., Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation, Nat. Chem. Biol, doi:10.1038/nchembio.1735
Li, Li, Lindberg, Kennett, Xiong et al., PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps, J. Exp. Med, doi:10.1084/jem.20100239
Li, Wu, Huang, Cao, An et al., A variety of death modes of neutrophils and their role in the etiology of autoimmune diseases, Immunol. Rev, doi:10.1111/imr.13284
Lingel, Meltendorf, Billing, Thurm, Vogel et al., Unique autoantibody prevalence in long-term recovered SARS-CoV-2infected individuals, J. Autoimmun, doi:10.1016/j.jaut.2021.102682
Liu, Kaplan, Neutrophils in the pathogenesis of rheumatic diseases: fueling the fire, Clin. Rev. Allergy Immunol, doi:10.1007/s12016-020-08816-3
Liu, Mak, Su, Yeoh, Lui et al., Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome, Gut, doi:10.1136/gutjnl-2021-325989
Lucchese, Flöel, SARS-CoV-2 and Guillain-Barré syndrome: molecular mimicry with human heat shock proteins as potential pathogenic mechanism, Cell Stress Chaperones, doi:10.1007/s12192-020-01145-6
Manolis, Manolis, Manolis, Papatheou, Melita, COVID-19 infection: viral macro-and micro-vascular coagulopathy and thromboembolism/ prophylactic and therapeutic management, J. Cardiovasc. Pharmacol. Therapeut, doi:10.1177/1074248420958973
Manry, Bastard, Gervais, Voyer, Rosain et al., None, doi:10.1073/pnas.2200413119
Mantovani Cardoso, Hundal, Feterman, Magaldi, Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology, Clin. Rheumatol, doi:10.1007/s10067-020-05310-1
Marín, Mazenett-Granados, Salazar-Uribe, Sarmiento, Suárez et al., Increased incidence of rheumatoid arthritis after COVID-19, Autoimmun. Rev, doi:10.1016/j.autrev.2023.103409
Mateu-Salat, Urgell, Chico, SARS-COV-2 as a trigger for autoimmune disease: report of two cases of Graves' disease after COVID-19, J. Endocrinol. Investig, doi:10.1007/s40618-020-01366-7
Mavragani, Moutsopoulos, Sjögren Syndrome, Can, None, Med. Assoc. J. = J. l'Association Medicale Can, doi:10.1503/cmaj.122037
Menegazzo, Scattolini, Cappellari, Bonora, Albiero et al., The antidiabetic drug metformin blunts NETosis in vitro and reduces circulating NETosis biomarkers in vivo, Acta Diabetol, doi:10.1007/s00592-018-1129-8
Middleton, He, Denorme, Campbell, Ng et al., Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome, Blood, doi:10.1182/blood.2020007008
Mobasheri, Nasirpour, Masoumi, Azarnaminy, Jafari et al., SARS-CoV-2 triggering autoimmune diseases, Cytokine, doi:10.1016/j.cyto.2022.155873
Monsalve, Acosta-Ampudia, Ramírez-Santana, Polo, Anaya, Neutrophil extracellular traps in autoimmune diseases, Rev. Colomb. Reumatol, doi:10.1016/j.rcreu.2020.04.007
Moore, Juo, Nielsen, Tyden, Bengtsson et al., Role of neutrophil extracellular traps regarding patients at risk of increased disease activity and cardiovascular comorbidity in systemic lupus erythematosus, J. Rheumatol, doi:10.3899/jrheum.190875
Moreira-Teixeira, Stimpson, Stavropoulos, Hadebe, Chakravarty et al., Type I IFN exacerbates disease in tuberculosis-susceptible mice by inducing neutrophil-mediated lung inflammation and NETosis, Nat. Commun, doi:10.1038/s41467-020-19412-6
Moreno-Pérez, Merino, Leon-Ramirez, Andres, Ramos et al., Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study, J. Infect, doi:10.1016/j.jinf.2021.01.004
Mutua, Gershwin, A review of neutrophil extracellular traps (NETs) in disease: potential anti-NETs therapeutics, Clin. Rev. Allergy Immunol, doi:10.1007/s12016-020-08804-7
Nakazawa, Shida, Tomaru, Yoshida, Nishio et al., Enhanced Formation and disordered regulation of NETs in myeloperoxidase-ANCA-associated microscopic polyangiitis, J. Am. Soc. Nephrol, doi:10.1681/ASN.2013060606
Nappi, Bellomo, Singh, Insights into the role of neutrophils and neutrophil extracellular traps in causing cardiovascular complications in patients with COVID-19: a systematic review, J. Clin. Med, doi:10.3390/jcm11092460
Negrini, Guadagno, Greco, Parodi, Burlando, An unusual case of bullous haemorrhagic vasculitis in a COVID-19 patient, J. Eur. Acad. Dermatol. Venereol, doi:10.1111/jdv.16760
Newton, Cardani, Braciale, The host immune response in respiratory virus infection: balancing virus clearance and immunopathology, Semin. Immunopathol, doi:10.1007/s00281-016-0558-0
Nicolai, Leunig, Brambs, Kaiser, Weinberger et al., Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy, Circulation, doi:10.1161/CIRCULATIONAHA.120.048488
Njeim, Azar, Fares, Azar, Kassouf et al., NETosis contributes to the pathogenesis of diabetes and its complications, J. Mol. Endocrinol, doi:10.1530/JME-20-0128
Nolan, Chenming, Shi-You, Transforming growth factor-β signaling in systemic sclerosis, J. Biomed. Res, doi:10.7555/JBR.31.20170034
Organization, WHO Coronavirus (COVID-19) Dashboard
Palao, Fernández-Díaz, Gracia-Gil, Romero-Sánchez, Díaz-Maroto et al., Multiple sclerosis following SARS-CoV-2 infection, Mult. Scler. Relat. Disord, doi:10.1016/j.msard.2020.102377
Papayannopoulos, Metzler, Hakkim, Zychlinsky, Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps, J. Cell Biol, doi:10.1083/jcb.201006052
Papayannopoulos, Neutrophil extracellular traps in immunity and disease, Nat. Rev. Immunol, doi:10.1038/nri.2017.105
Pascolini, Vannini, Deleonardi, Ciordinik, Sensoli et al., COVID-19 and immunological dysregulation: can autoantibodies be useful?, Clin. Transl. Sci, doi:10.1111/cts.12908
Peng, Wu, Zhang, Deng, Zhao et al., The potential roles of type I interferon activated neutrophils and neutrophil extracellular traps (NETs) in the pathogenesis of primary Sjögren's syndrome, Arthritis Res. Ther, doi:10.1186/s13075-022-02860-4
Pilsczek, Salina, Poon, Fahey, Yipp et al., A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus, J. Immunol, doi:10.4049/jimmunol.1000675
Pisareva, Badiou, Mihalovičová, Mirandola, Pastor et al., Persistence of neutrophil extracellular traps and anticardiolipin auto-antibodies in post-acute phase COVID-19 patients, J. Med. Virol, doi:10.1002/jmv.28209
Pramitasuri, Laksmidewi, Putra, Dalimartha, Neutrophil extracellular traps in coronavirus disease-19-associated ischemic stroke: a novel avenue in neuroscience, Exp. Neurobiol, doi:10.5607/en20048
Proal, Vanelzakker, Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms, Front. Microbiol, doi:10.3389/fmicb.2021.698169
Ravindran, Khan, Palaniyar, Neutrophil extracellular trap formation: physiology, pathology, and pharmacology, Biomolecules, doi:10.3390/biom9080365
Rojas, Herrán, Ramírez-Santana, Leung, Anaya et al., Molecular mimicry and autoimmunity in the time of COVID-19, J. Autoimmun, doi:10.1016/j.jaut.2023.103070
Rojas, Rodríguez, Acosta-Ampudia, Monsalve, Zhu et al., Autoimmunity is a hallmark of post-COVID syndrome, J. Transl. Med, doi:10.1186/s12967-022-03328-4
Rosazza, Warner, Sollberger, NET formation -mechanisms and how they relate to other cell death pathways, FEBS J, doi:10.1111/febs.15589
Rother, Pieterse, Lubbers, Hilbrands, Van Der et al., Acetylated histones in apoptotic microparticles drive the formation of neutrophil extracellular traps in active lupus nephritis, Front. Immunol, doi:10.3389/fimmu.2017.01136
Sadeghi, Dehnavi, Jamialahmadi, Johnston, Sahebkar, Neutrophil extracellular trap: a key player in the pathogenesis of autoimmune diseases, Int. Immunopharmacol, doi:10.1016/j.intimp.2023.109843
Salemme, Peralta, Meka, Pushpanathan, Alexander, The role of NETosis in systemic lupus erythematosus, J. Cell. Immunol, doi:10.33696/immunology.1.008
Salet, Bekkering, Middeldorp, Van Den Hoogen, Targeting thromboinflammation in antiphospholipid syndrome, J. Thromb. Haemostasis, doi:10.1016/j.jtha.2022.12.002
Sawadogo, Dighero-Kemp, Ouédraogo, Hensley, Sakandé, How NETosis could drive "Post-COVID-19 syndrome" among survivors, Immunol. Lett, doi:10.1016/j.imlet.2020.09.005
Seeßle, Waterboer, Hippchen, Simon, Kirchner et al., Persistent symptoms in adult patients 1 Year after coronavirus disease 2019 (COVID-19): a prospective cohort study, Clin. Infect. Dis. an Off. Publ. Infect. Dis. Soc. Am, doi:10.1093/cid/ciab611
Shafqat, Noor Eddin, Adi, Al-Rimawi, Abdul Rab et al., Neutrophil extracellular traps in central nervous system pathologies: a mini review, Front. Med, doi:10.3389/fmed.2023.1083242
Shafqat, Omer, Albalkhi, Alabdul Razzak, Abdulkader et al., Neutrophil extracellular traps and long COVID, Front. Immunol, doi:10.3389/fimmu.2023.1254310
Sherif, Gomez, Connors, Henrich, Reeves, Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC), Elife, doi:10.7554/eLife.86002
Shoenfeld, Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning, Autoimmun. Rev, doi:10.1016/j.autrev.2020.102538
Silva, Wanderley, Veras, Gonçalves, Lima et al., Gasdermin-D activation by SARS-CoV-2 triggers NET and mediate COVID-19 immunopathology, Crit. Care, doi:10.1186/s13054-022-04062-5
Skendros, Mitsios, Chrysanthopoulou, Mastellos, Metallidis et al., Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis, J. Clin. Investig, doi:10.1172/JCI141374
Soheili, Khateri, Moradpour, Mohammadzedeh, Zareie et al., The efficacy and effectiveness of COVID-19 vaccines around the world: a mini-review and meta-analysis, Ann. Clin. Microbiol. Antimicrob, doi:10.1186/s12941-023-00594-y
Sollini, Ciccarelli, Cecconi, Aghemo, Morelli et al., Vasculitis changes in COVID-19 survivors with persistent symptoms: an [(18)F] FDG-PET/CT study, Eur. J. Nucl. Med. Mol. Imag, doi:10.1007/s00259-020-05084-3
Song, Ye, Pan, Tan, Herrmann, Neutrophil extracellular traps tied to rheumatoid arthritis: points to ponder, Front. Immunol, doi:10.3389/fimmu.2020.578129
Soriano, Murthy, Marshall, Relan, Diaz, A clinical case definition of post-COVID-19 condition by a Delphi consensus, Lancet Infect. Dis, doi:10.1016/S1473-3099(21)00703-9
Spudich, Nath, Nervous system consequences of COVID-19, Science, doi:10.1126/science.abm2052
Su, Yuan, Chen, Ng, Wang et al., Multiple early factors anticipate post-acute COVID-19 sequelae, Cell, doi:10.1016/j.cell.2022.01.014
Sungnak, Huang, Bécavin, Berg, Queen et al., SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat. Med, doi:10.1038/s41591-020-0868-6
Swank, Senussi, Manickas-Hill, Yu, Li et al., Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae, Clin. Infect. Dis. an Off. Publ. Infect. Dis. Soc. Am, doi:10.1093/cid/ciac722
Sørensen, Spiliopoulos, Bager, Nielsen, Hansen et al., A nationwide questionnaire study of post-acute symptoms and health problems after SARS-CoV-2 infection in Denmark, Nat. Commun, doi:10.1038/s41467-022-31897-x
Tagami, Tosa, Omura, Fukushima, Kaneko et al., Effect of a selective neutrophil elastase inhibitor on mortality and ventilator-free days in patients with increased extravascular lung water: a post hoc analysis of the PiCCO Pulmonary Edema Study, J. Intensive Care, doi:10.1186/s40560-014-0067-y
Taghadosi, Safarzadeh, Asgarzadeh, Roghani, Shamsi et al., Partners in crime: autoantibodies complicit in COVID-19 pathogenesis, Rev. Med. Virol, doi:10.1002/rmv.2412
Tan, Aziz, Wang, The vitals of NETs, J. Leukoc. Biol, doi:10.1002/JLB.3RU0620-375R
Teodoro, Rodrigues, Farnesi-De-Assunção, Borges, Obata et al., Inflammatory response and activation of coagulation after COVID-19 infection, Viruses, doi:10.3390/v15040938
Tesch, Ehm, Vivirito, Wende, Batram et al., Incident autoimmune diseases in association with SARS-CoV-2 infection: a matched cohort study, Clin. Rheumatol, doi:10.1007/s10067-023-06670-0
Thierry, Salmon, Inflammation-, immunothrombosis,-and autoimmunefeedback loops may lead to persistent neutrophil self-stimulation in long COVID, J. Med. Virol, doi:10.1002/jmv.29887
Torres-Ruiz, Absalón-Aguilar, Nuñez-Aguirre, Pérez-Fragoso, Carrillo-Vázquez et al., Neutrophil extracellular traps contribute to COVID-19 hyperinflammation and humoral autoimmunity, Cells, doi:10.3390/cells10102545
Toscano, Palmerini, Ravaglia, Ruiz, Invernizzi et al., Guillain-barré syndrome associated with SARS-CoV-2, N. Engl. J. Med, doi:10.1056/NEJMc2009191
Tsampasian, Elghazaly, Chattopadhyay, Debski, Naing et al., Risk factors associated with post-COVID-19 condition: a systematic review and meta-analysis, JAMA Intern. Med, doi:10.1001/jamainternmed.2023.0750
Vahabi, Ghazanfari, Sepehrnia, Molecular mimicry, hyperactive immune system, and SARS-COV-2 are three prerequisites of the autoimmune disease triangle following COVID-19 infection, Int. Immunopharmacol, doi:10.1016/j.intimp.2022.109183
Van Den Hoogen, Khanna, Fransen, Johnson, Baron et al., classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative, Arthritis Rheum, doi:10.1002/art.38098
Vecchio, Lo Buono, Stabilini, Nigi, Dufort et al., Abnormal neutrophil signature in the blood and pancreas of presymptomatic and symptomatic type 1 diabetes, JCI Insight, doi:10.1172/jci.insight.122146
Venkatesan, NICE guideline on long COVID, Lancet Respir. Med, doi:10.1016/S2213-2600(21)00031-X
Ventura-Santana, Ninan, Snyder, Okeke, Neutrophil extracellular traps, sepsis and COVID-19 -a tripod stand, Front. Immunol, doi:10.3389/fimmu.2022.902206
Veras, Pontelli, Silva, Toller-Kawahisa, De Lima et al., SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology, J. Exp. Med, doi:10.1084/jem.20201129
Viner, Whittaker, Kawasaki-like disease: emerging complication during the COVID-19 pandemic, Lancet, doi:10.1016/S0140-6736(20)31129-6
Vlachoyiannopoulos, Magira, Alexopoulos, Jahaj, Theophilopoulou et al., Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19, Ann. Rheum. Dis, doi:10.1136/annrheumdis-2020-218009
Vojdani, Kharrazian, Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases, Clin. Immunol, doi:10.1016/j.clim.2020.108480
Von Scheven, Lu, Emery, Elder, Wara, Thrombosis and pediatric Wegener's granulomatosis: acquired and genetic risk factors for hypercoagulability, Arthritis Rheum, doi:10.1002/art.11454
Wang, Li, Yin, Zhang, Cao et al., Excessive neutrophils and neutrophil extracellular traps in COVID-19, Front. Immunol, doi:10.3389/fimmu.2020.02063
Wang, Mao, Klein, Dai, Huck et al., Diverse functional autoantibodies in patients with COVID-19, Nature, doi:10.1038/s41586-021-03631-y
Wang, Su, Yan, Pan, Zhang, Neutrophil extracellular traps in autoimmune diseases: analysis of the knowledge map, Front. Immunol, doi:10.3389/fimmu.2023.1095421
Wang, Xiao, Zhong, Ye, Zhang et al., Increased neutrophil elastase and proteinase 3 and augmented NETosis are closely associated with β-cell autoimmunity in patients with type 1 diabetes, Diabetes, doi:10.2337/db14-0480
Watad, Bragazzi, Amital, Shoenfeld, Hyperstimulation of adaptive immunity as the common pathway for silicone breast implants, autoimmunity, and lymphoma of the breast, Isr. Med. Assoc. J
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19), JAMA, doi:10.1001/jama.2020.12839
Woodruff, Bonham, Anam, Walker, Faliti et al., Chronic inflammation, neutrophil activity, and autoreactivity splits long COVID, Nat. Commun, doi:10.1038/s41467-023-40012-7
Xiang, Zhang, Jiang, Su, Shi, The role of inflammation in autoimmune disease: a therapeutic target, Front. Immunol, doi:10.3389/fimmu.2023.1267091
Xie, Xu, Bowe, Al-Aly, Long-term cardiovascular outcomes of COVID-19, Nat. Med, doi:10.1038/s41591-022-01689-3
Yalavarthi, Gould, Rao, Mazza, Morris et al., Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome, Arthritis Rheumatol, doi:10.1002/art.39247
Yang, Biermann, Brauner, Liu, Zhao et al., New insights into neutrophil extracellular traps: mechanisms of formation and role in inflammation, Front. Immunol, doi:10.3389/fimmu.2016.00302
Yao, Liu, Jallal, Shen, Rönnblom, Type I interferons in Sjögren's syndrome, Autoimmun. Rev, doi:10.1016/j.autrev.2012.10.006
Yipp, Kubes, NETosis: how vital is it?, Blood, doi:10.1182/blood-2013-04-457671
Yousefi, Simon, Stojkov, Karsonova, Karaulov et al., In vivo evidence for extracellular DNA trap formation, Cell Death Dis, doi:10.1038/s41419-020-2497-x
Zanin, Saraceno, Panciani, Renisi, Signorini et al., SARS-CoV-2 can induce brain and spine demyelinating lesions, Acta Neurochir, doi:10.1007/s00701-020-04374-x
Zanini, Selleri, Roncati, Coppi, Nasi et al., Vascular "long COVID": a new vessel disease?, Angiology, doi:10.1177/00033197231153204
Zhang, Bastard, Cobat, Casanova, Human genetic and immunological determinants of critical COVID-19 pneumonia, Nature, doi:10.1038/s41586-022-04447-0
Zhang, Xiao, Zhang, Xia, Cao et al., Coagulopathy and antiphospholipid antibodies in patients with covid-19, N. Engl. J. Med, doi:10.1056/NEJMc2007575
Zhang, Zhou, Zhu, Song, Feng et al., Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19, Front. Mol. Biosci, doi:10.3389/fmolb.2020.00157
Zhao, Shen, Zhou, Liu, Chen, Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence?, Lancet Neurol, doi:10.1016/S1474-4422(20)30109-5
Zheng, SARS-CoV-2: an emerging coronavirus that causes a global threat, Int. J. Biol. Sci, doi:10.7150/ijbs.45053
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
Zhu, Chen, Liu, NETosis and neutrophil extracellular traps in COVID-19: immunothrombosis and beyond, Front. Immunol, doi:10.3389/fimmu.2022.838011
Zulfiqar, Lorenzo-Villalba, Hassler, Andrès, Immune thrombocytopenic purpura in a patient with covid-19, N. Engl. J. Med, doi:10.1056/NEJMc2010472
Zuo, Navaz, Tsodikov, Kmetova, Kluge et al., Anti-neutrophil extracellular trap antibodies in antiphospholipid antibody-positive patients: results from the antiphospholipid syndrome alliance for clinical trials and <scp>InternatiOnal</scp> networking clinical database and repository, Arthritis Rheumatol, doi:10.1002/art.42489
Zuo, Yalavarthi, Gockman, Madison, Gudjonsson et al., Antineutrophil extracellular trap antibodies and impaired neutrophil extracellular trap degradation in antiphospholipid syndrome, Arthritis Rheumatol, doi:10.1002/art.41460
Zuo, Yalavarthi, Shi, Gockman, Zuo et al., Neutrophil extracellular traps in COVID-19, JCI Insight, doi:10.1172/jci.insight.138999
DOI record:
{
"DOI": "10.1016/j.jtauto.2025.100280",
"ISSN": [
"2589-9090"
],
"URL": "http://dx.doi.org/10.1016/j.jtauto.2025.100280",
"alternative-id": [
"S2589909025000152"
],
"article-number": "100280",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "NETosis: A key player in autoimmunity, COVID-19, and long COVID"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Translational Autoimmunity"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jtauto.2025.100280"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2025 Published by Elsevier B.V."
}
],
"author": [
{
"affiliation": [],
"family": "Monsalve",
"given": "Diana M.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Acosta-Ampudia",
"given": "Yeny",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Acosta",
"given": "Nicolás Guerrero",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Celis-Andrade",
"given": "Mariana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Şahin",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yilmaz",
"given": "Ahsen Morva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shoenfeld",
"given": "Yehuda",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2137-4899",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ramírez-Santana",
"given": "Carolina",
"sequence": "additional"
}
],
"container-title": "Journal of Translational Autoimmunity",
"container-title-short": "Journal of Translational Autoimmunity",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2025,
2,
21
]
],
"date-time": "2025-02-21T16:39:21Z",
"timestamp": 1740155961000
},
"deposited": {
"date-parts": [
[
2025,
3,
8
]
],
"date-time": "2025-03-08T23:31:24Z",
"timestamp": 1741476684000
},
"funder": [
{
"DOI": "10.13039/501100008793",
"award": [
"ABN-011",
"IV-PBG005"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100008793",
"id-type": "DOI"
}
],
"name": "University of Rosario"
}
],
"indexed": {
"date-parts": [
[
2025,
3,
9
]
],
"date-time": "2025-03-09T05:10:18Z",
"timestamp": 1741497018375,
"version": "3.38.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
6,
1
]
],
"date-time": "2025-06-01T00:00:00Z",
"timestamp": 1748736000000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
6,
1
]
],
"date-time": "2025-06-01T00:00:00Z",
"timestamp": 1748736000000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
2,
21
]
],
"date-time": "2025-02-21T00:00:00Z",
"timestamp": 1740096000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2589909025000152?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2589909025000152?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100280",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2025,
6
]
]
},
"published-print": {
"date-parts": [
[
2025,
6
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "Circulating neutrophil extracellular trap (NET)-forming “rogue” neutrophil subset, immunotype [DEspR+CD11b+], mediate multi-organ failure in COVID-19 - an observational study",
"author": "Herrera",
"journal-title": "Res. Sq",
"key": "10.1016/j.jtauto.2025.100280_bib1",
"year": "2023"
},
{
"DOI": "10.1038/nri.2017.105",
"article-title": "Neutrophil extracellular traps in immunity and disease",
"author": "Papayannopoulos",
"doi-asserted-by": "crossref",
"first-page": "134",
"journal-title": "Nat. Rev. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib2",
"volume": "18",
"year": "2018"
},
{
"DOI": "10.1038/nm.4294",
"article-title": "An emerging role for neutrophil extracellular traps in noninfectious disease",
"author": "Jorch",
"doi-asserted-by": "crossref",
"first-page": "279",
"journal-title": "Nat. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib3",
"volume": "23",
"year": "2017"
},
{
"DOI": "10.1007/s12016-020-08804-7",
"article-title": "A review of neutrophil extracellular traps (NETs) in disease: potential anti-NETs therapeutics",
"author": "Mutua",
"doi-asserted-by": "crossref",
"first-page": "194",
"journal-title": "Clin. Rev. Allergy Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib4",
"volume": "61",
"year": "2021"
},
{
"DOI": "10.1016/j.intimp.2023.109843",
"article-title": "Neutrophil extracellular trap: a key player in the pathogenesis of autoimmune diseases",
"author": "Sadeghi",
"doi-asserted-by": "crossref",
"journal-title": "Int. Immunopharmacol.",
"key": "10.1016/j.jtauto.2025.100280_bib5",
"volume": "116",
"year": "2023"
},
{
"DOI": "10.1038/s41598-021-95209-x",
"article-title": "Evolution of NETosis markers and DAMPs have prognostic value in critically ill COVID-19 patients",
"author": "Huckriede",
"doi-asserted-by": "crossref",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.jtauto.2025.100280_bib6",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.ebiom.2020.102925",
"article-title": "Vascular occlusion by neutrophil extracellular traps in COVID-19",
"author": "Leppkes",
"doi-asserted-by": "crossref",
"journal-title": "EBioMedicine",
"key": "10.1016/j.jtauto.2025.100280_bib7",
"volume": "58",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2022.902206",
"article-title": "Neutrophil extracellular traps, sepsis and COVID-19 – a tripod stand",
"author": "Ventura-Santana",
"doi-asserted-by": "crossref",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib8",
"volume": "13",
"year": "2022"
},
{
"key": "10.1016/j.jtauto.2025.100280_bib9",
"series-title": "WHO Coronavirus (COVID-19) Dashboard",
"year": "2023"
},
{
"DOI": "10.1001/jama.2020.12839",
"article-title": "Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19)",
"author": "Wiersinga",
"doi-asserted-by": "crossref",
"first-page": "782",
"journal-title": "JAMA",
"key": "10.1016/j.jtauto.2025.100280_bib10",
"volume": "324",
"year": "2020"
},
{
"article-title": "Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis",
"author": "Alkodaymi",
"first-page": "657",
"journal-title": "Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis.",
"key": "10.1016/j.jtauto.2025.100280_bib11",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(21)00703-9",
"article-title": "A clinical case definition of post-COVID-19 condition by a Delphi consensus",
"author": "Soriano",
"doi-asserted-by": "crossref",
"first-page": "e102",
"journal-title": "Lancet Infect. Dis.",
"key": "10.1016/j.jtauto.2025.100280_bib12",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1136/bmj.n693",
"article-title": "Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study",
"author": "Ayoubkhani",
"doi-asserted-by": "crossref",
"first-page": "n693",
"journal-title": "BMJ",
"key": "10.1016/j.jtauto.2025.100280_bib13",
"volume": "372",
"year": "2021"
},
{
"DOI": "10.1016/j.jtha.2023.02.033",
"article-title": "NETosis induction reflects COVID-19 severity and long COVID: insights from a 2-center patient cohort study in Israel",
"author": "Krinsky",
"doi-asserted-by": "crossref",
"journal-title": "J. Thromb. Haemostasis",
"key": "10.1016/j.jtauto.2025.100280_bib14",
"year": "2023"
},
{
"DOI": "10.1016/j.immuni.2021.06.006",
"article-title": "The neutrophil",
"author": "Burn",
"doi-asserted-by": "crossref",
"first-page": "1377",
"journal-title": "Immunity",
"key": "10.1016/j.jtauto.2025.100280_bib15",
"volume": "54",
"year": "2021"
},
{
"DOI": "10.3390/biom9080365",
"article-title": "Neutrophil extracellular trap formation: physiology, pathology, and pharmacology",
"author": "Ravindran",
"doi-asserted-by": "crossref",
"journal-title": "Biomolecules",
"key": "10.1016/j.jtauto.2025.100280_bib16",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1038/s41419-020-2497-x",
"article-title": "In vivo evidence for extracellular DNA trap formation",
"author": "Yousefi",
"doi-asserted-by": "crossref",
"first-page": "300",
"journal-title": "Cell Death Dis.",
"key": "10.1016/j.jtauto.2025.100280_bib17",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1038/s41423-018-0024-0",
"article-title": "NETosis, complement, and coagulation: a triangular relationship",
"author": "de Bont",
"doi-asserted-by": "crossref",
"first-page": "19",
"journal-title": "Cell. Mol. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib18",
"volume": "16",
"year": "2019"
},
{
"DOI": "10.1182/blood-2018-11-844530",
"article-title": "Neutrophils and NETs in modulating acute and chronic inflammation",
"author": "Castanheira",
"doi-asserted-by": "crossref",
"first-page": "2178",
"journal-title": "Blood",
"key": "10.1016/j.jtauto.2025.100280_bib19",
"volume": "133",
"year": "2019"
},
{
"DOI": "10.1038/s41418-018-0261-x",
"article-title": "To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps",
"author": "Boeltz",
"doi-asserted-by": "crossref",
"first-page": "395",
"journal-title": "Cell Death Differ.",
"key": "10.1016/j.jtauto.2025.100280_bib20",
"volume": "26",
"year": "2019"
},
{
"DOI": "10.4049/jimmunol.1000675",
"article-title": "A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus",
"author": "Pilsczek",
"doi-asserted-by": "crossref",
"first-page": "7413",
"journal-title": "J. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib21",
"volume": "185",
"year": "2010"
},
{
"DOI": "10.1038/nchembio.496",
"article-title": "Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation",
"author": "Hakkim",
"doi-asserted-by": "crossref",
"first-page": "75",
"journal-title": "Nat. Chem. Biol.",
"key": "10.1016/j.jtauto.2025.100280_bib22",
"volume": "7",
"year": "2011"
},
{
"DOI": "10.3389/fimmu.2016.00302",
"article-title": "New insights into neutrophil extracellular traps: mechanisms of formation and role in inflammation",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "302",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib23",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1084/jem.20100239",
"article-title": "PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "1853",
"journal-title": "J. Exp. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib24",
"volume": "207",
"year": "2010"
},
{
"DOI": "10.1083/jcb.201006052",
"article-title": "Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps",
"author": "Papayannopoulos",
"doi-asserted-by": "crossref",
"first-page": "677",
"journal-title": "J. Cell Biol.",
"key": "10.1016/j.jtauto.2025.100280_bib25",
"volume": "191",
"year": "2010"
},
{
"DOI": "10.1038/nchembio.1735",
"article-title": "Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation",
"author": "Lewis",
"doi-asserted-by": "crossref",
"first-page": "189",
"journal-title": "Nat. Chem. Biol.",
"key": "10.1016/j.jtauto.2025.100280_bib26",
"volume": "11",
"year": "2015"
},
{
"DOI": "10.1002/JLB.3RU0620-375R",
"article-title": "The vitals of NETs",
"author": "Tan",
"doi-asserted-by": "crossref",
"first-page": "797",
"journal-title": "J. Leukoc. Biol.",
"key": "10.1016/j.jtauto.2025.100280_bib27",
"volume": "110",
"year": "2021"
},
{
"DOI": "10.1182/blood-2013-04-457671",
"article-title": "NETosis: how vital is it?",
"author": "Yipp",
"doi-asserted-by": "crossref",
"first-page": "2784",
"journal-title": "Blood",
"key": "10.1016/j.jtauto.2025.100280_bib28",
"volume": "122",
"year": "2013"
},
{
"DOI": "10.1111/febs.15589",
"article-title": "NET formation - mechanisms and how they relate to other cell death pathways",
"author": "Rosazza",
"doi-asserted-by": "crossref",
"first-page": "3334",
"journal-title": "FEBS J.",
"key": "10.1016/j.jtauto.2025.100280_bib29",
"volume": "288",
"year": "2021"
},
{
"DOI": "10.1007/s12026-016-8817-7",
"article-title": "HLA-DRB1 the notorious gene in the mosaic of autoimmunity",
"author": "Arango",
"doi-asserted-by": "crossref",
"first-page": "82",
"journal-title": "Immunol. Res.",
"key": "10.1016/j.jtauto.2025.100280_bib30",
"volume": "65",
"year": "2017"
},
{
"article-title": "Hyperstimulation of adaptive immunity as the common pathway for silicone breast implants, autoimmunity, and lymphoma of the breast",
"author": "Watad",
"first-page": "517",
"journal-title": "Isr. Med. Assoc. J.",
"key": "10.1016/j.jtauto.2025.100280_bib31",
"volume": "21",
"year": "2019"
},
{
"article-title": "Neutrophil extracellular traps in autoimmune diseases: analysis of the knowledge map",
"author": "Wang",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib32",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1111/imr.13284",
"article-title": "A variety of death modes of neutrophils and their role in the etiology of autoimmune diseases",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "280",
"journal-title": "Immunol. Rev.",
"key": "10.1016/j.jtauto.2025.100280_bib33",
"volume": "321",
"year": "2024"
},
{
"DOI": "10.1002/art.41417",
"article-title": "Rheumatoid arthritis pathogenesis, prediction, and prevention: an emerging paradigm shift",
"author": "Deane",
"doi-asserted-by": "crossref",
"first-page": "181",
"journal-title": "Arthritis Rheumatol.",
"key": "10.1016/j.jtauto.2025.100280_bib34",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1126/scitranslmed.3005580",
"article-title": "NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis",
"author": "Khandpur",
"doi-asserted-by": "crossref",
"journal-title": "Sci. Transl. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib35",
"volume": "5",
"year": "2013"
},
{
"DOI": "10.3389/fimmu.2020.578129",
"article-title": "Neutrophil extracellular traps tied to rheumatoid arthritis: points to ponder",
"author": "Song",
"doi-asserted-by": "crossref",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib36",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1186/ar4579",
"article-title": "Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility",
"author": "Chowdhury",
"doi-asserted-by": "crossref",
"first-page": "R122",
"journal-title": "Arthritis Res. Ther.",
"key": "10.1016/j.jtauto.2025.100280_bib37",
"volume": "16",
"year": "2014"
},
{
"article-title": "An overview of systemic lupus erythematosus (SLE) pathogenesis, classification, and management",
"author": "Ameer",
"journal-title": "Cureus",
"key": "10.1016/j.jtauto.2025.100280_bib38",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.4049/jimmunol.1102404",
"article-title": "Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease",
"author": "Leffler",
"doi-asserted-by": "crossref",
"first-page": "3522",
"journal-title": "J. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib39",
"volume": "188",
"year": "2012"
},
{
"article-title": "The role of NETosis in systemic lupus erythematosus",
"author": "Salemme",
"first-page": "33",
"journal-title": "J. Cell. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib40",
"volume": "1",
"year": "2019"
},
{
"DOI": "10.1073/pnas.0909927107",
"article-title": "Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis",
"author": "Hakkim",
"doi-asserted-by": "crossref",
"first-page": "9813",
"journal-title": "Proc. Natl. Acad. Sci.",
"key": "10.1016/j.jtauto.2025.100280_bib41",
"volume": "107",
"year": "2010"
},
{
"DOI": "10.1126/scitranslmed.3001180",
"article-title": "Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA–peptide complexes in systemic lupus erythematosus",
"author": "Lande",
"doi-asserted-by": "crossref",
"journal-title": "Sci. Transl. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib42",
"volume": "3",
"year": "2011"
},
{
"DOI": "10.3899/jrheum.190875",
"article-title": "Role of neutrophil extracellular traps regarding patients at risk of increased disease activity and cardiovascular comorbidity in systemic lupus erythematosus",
"author": "Moore",
"doi-asserted-by": "crossref",
"first-page": "1652",
"journal-title": "J. Rheumatol.",
"key": "10.1016/j.jtauto.2025.100280_bib43",
"volume": "47",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2017.01136",
"article-title": "Acetylated histones in apoptotic microparticles drive the formation of neutrophil extracellular traps in active lupus nephritis",
"author": "Rother",
"doi-asserted-by": "crossref",
"first-page": "1136",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib44",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1007/s00281-022-00916-w",
"article-title": "Mechanisms of immunothrombosis and vasculopathy in antiphospholipid syndrome",
"author": "Knight",
"doi-asserted-by": "crossref",
"first-page": "347",
"journal-title": "Semin. Immunopathol.",
"key": "10.1016/j.jtauto.2025.100280_bib45",
"volume": "44",
"year": "2022"
},
{
"DOI": "10.1016/j.jtha.2022.12.002",
"article-title": "Targeting thromboinflammation in antiphospholipid syndrome",
"author": "Salet",
"doi-asserted-by": "crossref",
"first-page": "744",
"journal-title": "J. Thromb. Haemostasis",
"key": "10.1016/j.jtauto.2025.100280_bib46",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1007/s12016-020-08816-3",
"article-title": "Neutrophils in the pathogenesis of rheumatic diseases: fueling the fire",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Clin. Rev. Allergy Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib47",
"volume": "60",
"year": "2021"
},
{
"DOI": "10.1016/j.thromres.2022.05.001",
"article-title": "Association between neutrophil extracellular traps (NETs) and thrombosis in antiphospholipid syndrome",
"author": "de Moraes Mazetto",
"doi-asserted-by": "crossref",
"first-page": "132",
"journal-title": "Thromb. Res.",
"key": "10.1016/j.jtauto.2025.100280_bib48",
"volume": "214",
"year": "2022"
},
{
"DOI": "10.1002/art.39247",
"article-title": "Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome",
"author": "Yalavarthi",
"doi-asserted-by": "crossref",
"first-page": "2990",
"journal-title": "Arthritis Rheumatol.",
"key": "10.1016/j.jtauto.2025.100280_bib49",
"volume": "67",
"year": "2015"
},
{
"DOI": "10.1002/art.41460",
"article-title": "Anti-neutrophil extracellular trap antibodies and impaired neutrophil extracellular trap degradation in antiphospholipid syndrome",
"author": "Zuo",
"doi-asserted-by": "crossref",
"first-page": "2130",
"journal-title": "Arthritis Rheumatol.",
"key": "10.1016/j.jtauto.2025.100280_bib50",
"volume": "72",
"year": "2020"
},
{
"DOI": "10.1002/art.42489",
"article-title": "Anti–neutrophil extracellular trap antibodies in antiphospholipid antibody–positive patients: results from the antiphospholipid syndrome alliance for clinical trials and <scp>InternatiOnal</scp> networking clinical database and repository",
"author": "Zuo",
"doi-asserted-by": "crossref",
"first-page": "1407",
"journal-title": "Arthritis Rheumatol.",
"key": "10.1016/j.jtauto.2025.100280_bib51",
"volume": "75",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(18)31320-5",
"article-title": "Type 1 diabetes",
"author": "DiMeglio",
"doi-asserted-by": "crossref",
"first-page": "2449",
"journal-title": "Lancet (London, England)",
"key": "10.1016/j.jtauto.2025.100280_bib52",
"volume": "391",
"year": "2018"
},
{
"DOI": "10.1172/jci.insight.122146",
"article-title": "Abnormal neutrophil signature in the blood and pancreas of presymptomatic and symptomatic type 1 diabetes",
"author": "Vecchio",
"doi-asserted-by": "crossref",
"journal-title": "JCI Insight",
"key": "10.1016/j.jtauto.2025.100280_bib53",
"volume": "3",
"year": "2018"
},
{
"DOI": "10.1530/JME-20-0128",
"article-title": "NETosis contributes to the pathogenesis of diabetes and its complications",
"author": "Njeim",
"doi-asserted-by": "crossref",
"first-page": "R65",
"journal-title": "J. Mol. Endocrinol.",
"key": "10.1016/j.jtauto.2025.100280_bib54",
"volume": "65",
"year": "2020"
},
{
"DOI": "10.2337/db14-0480",
"article-title": "Increased neutrophil elastase and proteinase 3 and augmented NETosis are closely associated with β-cell autoimmunity in patients with type 1 diabetes",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "4239",
"journal-title": "Diabetes",
"key": "10.1016/j.jtauto.2025.100280_bib55",
"volume": "63",
"year": "2014"
},
{
"DOI": "10.3389/fimmu.2021.799539",
"article-title": "NETosis in long-term type 1 diabetes mellitus and its link to coronary artery disease",
"author": "Aukrust",
"doi-asserted-by": "crossref",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib56",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1503/cmaj.122037",
"article-title": "Sjögren syndrome",
"author": "Mavragani",
"doi-asserted-by": "crossref",
"first-page": "E579",
"journal-title": "C. Can. Med. Assoc. J. = J. l’Association Medicale Can.",
"key": "10.1016/j.jtauto.2025.100280_bib57",
"volume": "186",
"year": "2014"
},
{
"DOI": "10.1016/j.autrev.2012.10.006",
"article-title": "Type I interferons in Sjögren’s syndrome",
"author": "Yao",
"doi-asserted-by": "crossref",
"first-page": "558",
"journal-title": "Autoimmun. Rev.",
"key": "10.1016/j.jtauto.2025.100280_bib58",
"volume": "12",
"year": "2013"
},
{
"DOI": "10.1186/s13075-022-02860-4",
"article-title": "The potential roles of type I interferon activated neutrophils and neutrophil extracellular traps (NETs) in the pathogenesis of primary Sjögren’s syndrome",
"author": "Peng",
"doi-asserted-by": "crossref",
"first-page": "170",
"journal-title": "Arthritis Res. Ther.",
"key": "10.1016/j.jtauto.2025.100280_bib59",
"volume": "24",
"year": "2022"
},
{
"article-title": "Neutrophil extracellular traps in autoimmune diseases",
"author": "Monsalve",
"first-page": "4",
"journal-title": "Rev. Colomb. Reumatol.",
"key": "10.1016/j.jtauto.2025.100280_bib60",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1007/s12016-022-08946-w",
"article-title": "Systemic sclerosis-specific antibodies: novel and classical biomarkers",
"author": "Cavazzana",
"doi-asserted-by": "crossref",
"first-page": "412",
"journal-title": "Clin. Rev. Allergy Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib61",
"volume": "64",
"year": "2022"
},
{
"DOI": "10.1002/art.38098",
"article-title": "2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative",
"author": "van den Hoogen",
"doi-asserted-by": "crossref",
"first-page": "2737",
"journal-title": "Arthritis Rheum.",
"key": "10.1016/j.jtauto.2025.100280_bib62",
"volume": "65",
"year": "2013"
},
{
"DOI": "10.7555/JBR.31.20170034",
"article-title": "Transforming growth factor-β signaling in systemic sclerosis",
"author": "Nolan",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "J. Biomed. Res.",
"key": "10.1016/j.jtauto.2025.100280_bib63",
"volume": "32",
"year": "2018"
},
{
"DOI": "10.1007/s00281-013-0384-6",
"article-title": "Molecular mechanisms regulating NETosis in infection and disease",
"author": "Branzk",
"doi-asserted-by": "crossref",
"first-page": "513",
"journal-title": "Semin. Immunopathol.",
"key": "10.1016/j.jtauto.2025.100280_bib64",
"volume": "35",
"year": "2013"
},
{
"DOI": "10.3390/jcm9072136",
"article-title": "Neutrophil extracellular traps generation relates with early stage and vascular complications in systemic sclerosis",
"author": "Didier",
"doi-asserted-by": "crossref",
"first-page": "2136",
"journal-title": "J. Clin. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib65",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.7150/ijms.53728",
"article-title": "Neutrophil extracellular traps (NETs) and vasculitis",
"author": "Arneth",
"doi-asserted-by": "crossref",
"first-page": "1532",
"journal-title": "Int. J. Med. Sci.",
"key": "10.1016/j.jtauto.2025.100280_bib66",
"volume": "18",
"year": "2021"
},
{
"article-title": "N-formyl methionine peptide-mediated neutrophil activation in systemic sclerosis",
"author": "Kuley",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib67",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41572-020-0204-y",
"article-title": "ANCA-associated vasculitis",
"author": "Kitching",
"doi-asserted-by": "crossref",
"first-page": "71",
"journal-title": "Nat. Rev. Dis. Primers",
"key": "10.1016/j.jtauto.2025.100280_bib68",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1073/pnas.87.11.4115",
"article-title": "Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro",
"author": "Falk",
"doi-asserted-by": "crossref",
"first-page": "4115",
"journal-title": "Proc. Natl. Acad. Sci.",
"key": "10.1016/j.jtauto.2025.100280_bib69",
"volume": "87",
"year": "1990"
},
{
"DOI": "10.1038/nm.1959",
"article-title": "Netting neutrophils in autoimmune small-vessel vasculitis",
"author": "Kessenbrock",
"doi-asserted-by": "crossref",
"first-page": "623",
"journal-title": "Nat. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib70",
"volume": "15",
"year": "2009"
},
{
"DOI": "10.1681/ASN.2013060606",
"article-title": "Enhanced Formation and disordered regulation of NETs in myeloperoxidase-ANCA–associated microscopic polyangiitis",
"author": "Nakazawa",
"doi-asserted-by": "crossref",
"first-page": "990",
"journal-title": "J. Am. Soc. Nephrol.",
"key": "10.1016/j.jtauto.2025.100280_bib71",
"volume": "25",
"year": "2014"
},
{
"DOI": "10.1002/art.11454",
"article-title": "Thrombosis and pediatric Wegener's granulomatosis: acquired and genetic risk factors for hypercoagulability",
"author": "Von Scheven",
"doi-asserted-by": "crossref",
"first-page": "862",
"journal-title": "Arthritis Rheum.",
"key": "10.1016/j.jtauto.2025.100280_bib72",
"volume": "49",
"year": "2003"
},
{
"DOI": "10.7150/ijbs.45053",
"article-title": "SARS-CoV-2: an emerging coronavirus that causes a global threat",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "1678",
"journal-title": "Int. J. Biol. Sci.",
"key": "10.1016/j.jtauto.2025.100280_bib73",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.3390/jcm9061753",
"article-title": "COVID-19: specific and non-specific clinical manifestations and symptoms: the current state of knowledge",
"author": "Baj",
"doi-asserted-by": "crossref",
"first-page": "1753",
"journal-title": "J. Clin. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib74",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1038/s41579-020-00459-7",
"article-title": "Characteristics of SARS-CoV-2 and COVID-19",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "141",
"journal-title": "Nat. Rev. Microbiol.",
"key": "10.1016/j.jtauto.2025.100280_bib75",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1016/j.autrev.2020.102538",
"article-title": "Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning",
"author": "Shoenfeld",
"doi-asserted-by": "crossref",
"journal-title": "Autoimmun. Rev.",
"key": "10.1016/j.jtauto.2025.100280_bib76",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1186/s12941-023-00594-y",
"article-title": "The efficacy and effectiveness of COVID-19 vaccines around the world: a mini-review and meta-analysis",
"author": "Soheili",
"doi-asserted-by": "crossref",
"first-page": "42",
"journal-title": "Ann. Clin. Microbiol. Antimicrob.",
"key": "10.1016/j.jtauto.2025.100280_bib77",
"volume": "22",
"year": "2023"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"article-title": "SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes",
"author": "Sungnak",
"doi-asserted-by": "crossref",
"first-page": "681",
"journal-title": "Nat. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib78",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1038/s41580-021-00418-x",
"article-title": "Mechanisms of SARS-CoV-2 entry into cells",
"author": "Jackson",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "Nat. Rev. Mol. Cell Biol.",
"key": "10.1016/j.jtauto.2025.100280_bib79",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-03570-8",
"article-title": "COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets",
"author": "Delorey",
"doi-asserted-by": "crossref",
"first-page": "107",
"journal-title": "Nature",
"key": "10.1016/j.jtauto.2025.100280_bib80",
"volume": "595",
"year": "2021"
},
{
"DOI": "10.3390/v15040938",
"article-title": "Inflammatory response and activation of coagulation after COVID-19 infection",
"author": "Teodoro",
"doi-asserted-by": "crossref",
"journal-title": "Viruses",
"key": "10.1016/j.jtauto.2025.100280_bib81",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1016/j.cyto.2022.155873",
"article-title": "SARS-CoV-2 triggering autoimmune diseases",
"author": "Mobasheri",
"doi-asserted-by": "crossref",
"journal-title": "Cytokine",
"key": "10.1016/j.jtauto.2025.100280_bib82",
"volume": "154",
"year": "2022"
},
{
"DOI": "10.1016/j.autrev.2020.102597",
"article-title": "Covid-19 and autoimmunity",
"author": "Ehrenfeld",
"doi-asserted-by": "crossref",
"journal-title": "Autoimmun. Rev.",
"key": "10.1016/j.jtauto.2025.100280_bib83",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1016/j.jtauto.2021.100091",
"article-title": "Latent rheumatic, thyroid and phospholipid autoimmunity in hospitalized patients with COVID-19",
"author": "Anaya",
"doi-asserted-by": "crossref",
"journal-title": "J. Transl. Autoimmun.",
"key": "10.1016/j.jtauto.2025.100280_bib84",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1016/j.autrev.2020.102695",
"article-title": "SARS-CoV-2, the autoimmune virus",
"author": "Halpert",
"doi-asserted-by": "crossref",
"journal-title": "Autoimmun. Rev.",
"key": "10.1016/j.jtauto.2025.100280_bib85",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1038/s41467-022-28905-5",
"article-title": "Autoantibodies targeting GPCRs and RAS-related molecules associate with COVID-19 severity",
"author": "Cabral-Marques",
"doi-asserted-by": "crossref",
"first-page": "1220",
"journal-title": "Nat. Commun.",
"key": "10.1016/j.jtauto.2025.100280_bib86",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1038/s41467-021-25509-3",
"article-title": "New-onset IgG autoantibodies in hospitalized patients with COVID-19",
"author": "Chang",
"doi-asserted-by": "crossref",
"first-page": "5417",
"journal-title": "Nat. Commun.",
"key": "10.1016/j.jtauto.2025.100280_bib87",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1136/annrheumdis-2020-218009",
"article-title": "Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19",
"author": "Vlachoyiannopoulos",
"doi-asserted-by": "crossref",
"first-page": "1661",
"journal-title": "Ann. Rheum. Dis.",
"key": "10.1016/j.jtauto.2025.100280_bib88",
"volume": "79",
"year": "2020"
},
{
"DOI": "10.1111/cts.12908",
"article-title": "COVID-19 and immunological dysregulation: can autoantibodies be useful?",
"author": "Pascolini",
"doi-asserted-by": "crossref",
"first-page": "502",
"journal-title": "Clin. Transl. Sci.",
"key": "10.1016/j.jtauto.2025.100280_bib89",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2007575",
"article-title": "Coagulopathy and antiphospholipid antibodies in patients with covid-19",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "e38",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib90",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.587517",
"article-title": "Clinical, serological, and histopathological similarities between severe COVID-19 and acute exacerbation of connective tissue disease-associated interstitial lung disease (CTD-ILD)",
"author": "Gagiannis",
"doi-asserted-by": "crossref",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib91",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1126/sciimmunol.abl4340",
"article-title": "Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths",
"author": "Bastard",
"doi-asserted-by": "crossref",
"journal-title": "Sci. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib92",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1038/s41586-022-04447-0",
"article-title": "Human genetic and immunological determinants of critical COVID-19 pneumonia",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "587",
"journal-title": "Nature",
"key": "10.1016/j.jtauto.2025.100280_bib93",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1073/pnas.2200413119",
"article-title": "The risk of COVID-19 death is much greater and age dependent with type I IFN autoantibodies",
"author": "Manry",
"doi-asserted-by": "crossref",
"journal-title": "Proc. Natl. Acad. Sci. U.S.A.",
"key": "10.1016/j.jtauto.2025.100280_bib94",
"volume": "119",
"year": "2022"
},
{
"DOI": "10.1371/journal.pbio.3001709",
"article-title": "Critically ill COVID-19 patients with neutralizing autoantibodies against type I interferons have increased risk of herpesvirus disease",
"author": "Busnadiego",
"doi-asserted-by": "crossref",
"journal-title": "PLoS Biol.",
"key": "10.1016/j.jtauto.2025.100280_bib95",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1007/s10067-020-05310-1",
"article-title": "Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology",
"author": "Mantovani Cardoso",
"doi-asserted-by": "crossref",
"first-page": "2811",
"journal-title": "Clin. Rheumatol.",
"key": "10.1016/j.jtauto.2025.100280_bib96",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1111/bjh.16794",
"article-title": "Autoimmune haemolytic anaemia associated with COVID-19 infection",
"author": "Lazarian",
"doi-asserted-by": "crossref",
"first-page": "29",
"journal-title": "Br. J. Haematol.",
"key": "10.1016/j.jtauto.2025.100280_bib97",
"volume": "190",
"year": "2020"
},
{
"DOI": "10.1007/s40618-020-01366-7",
"article-title": "SARS-COV-2 as a trigger for autoimmune disease: report of two cases of Graves' disease after COVID-19",
"author": "Mateu-Salat",
"doi-asserted-by": "crossref",
"first-page": "1527",
"journal-title": "J. Endocrinol. Investig.",
"key": "10.1016/j.jtauto.2025.100280_bib98",
"volume": "43",
"year": "2020"
},
{
"DOI": "10.1212/NXI.0000000000000741",
"article-title": "Guillain-Barré syndrome related to COVID-19 infection",
"author": "Alberti",
"doi-asserted-by": "crossref",
"journal-title": "Neurol. Neuroimmunol. Neuroinflammation.",
"key": "10.1016/j.jtauto.2025.100280_bib99",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2009191",
"article-title": "Guillain-barré syndrome associated with SARS-CoV-2",
"author": "Toscano",
"doi-asserted-by": "crossref",
"first-page": "2574",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib100",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S1474-4422(20)30109-5",
"article-title": "Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence?",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "383",
"journal-title": "Lancet Neurol.",
"key": "10.1016/j.jtauto.2025.100280_bib101",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1016/j.msard.2020.102377",
"article-title": "Multiple sclerosis following SARS-CoV-2 infection",
"author": "Palao",
"doi-asserted-by": "crossref",
"journal-title": "Mult. Scler. Relat. Disord.",
"key": "10.1016/j.jtauto.2025.100280_bib102",
"volume": "45",
"year": "2020"
},
{
"DOI": "10.1007/s00701-020-04374-x",
"article-title": "SARS-CoV-2 can induce brain and spine demyelinating lesions",
"author": "Zanin",
"doi-asserted-by": "crossref",
"first-page": "1491",
"journal-title": "Acta Neurochir.",
"key": "10.1016/j.jtauto.2025.100280_bib103",
"volume": "162",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2010472",
"article-title": "Immune thrombocytopenic purpura in a patient with covid-19",
"author": "Zulfiqar",
"doi-asserted-by": "crossref",
"first-page": "e43",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib104",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1002/art.41428",
"article-title": "COVID-19-Related IgA vasculitis",
"author": "Allez",
"doi-asserted-by": "crossref",
"first-page": "1952",
"journal-title": "Arthritis Rheumatol.",
"key": "10.1016/j.jtauto.2025.100280_bib105",
"volume": "72",
"year": "2020"
},
{
"DOI": "10.1111/jdv.16760",
"article-title": "An unusual case of bullous haemorrhagic vasculitis in a COVID-19 patient",
"author": "Negrini",
"doi-asserted-by": "crossref",
"first-page": "e675",
"journal-title": "J. Eur. Acad. Dermatol. Venereol.",
"key": "10.1016/j.jtauto.2025.100280_bib106",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1111/jdv.16763",
"article-title": "Catastrophic acute bilateral lower limbs necrosis associated with COVID-19 as a likely consequence of both vasculitis and coagulopathy",
"author": "Del Giudice",
"doi-asserted-by": "crossref",
"first-page": "e679",
"journal-title": "J. Eur. Acad. Dermatol. Venereol.",
"key": "10.1016/j.jtauto.2025.100280_bib107",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31129-6",
"article-title": "Kawasaki-like disease: emerging complication during the COVID-19 pandemic",
"author": "Viner",
"doi-asserted-by": "crossref",
"first-page": "1741",
"journal-title": "Lancet (London, England)",
"key": "10.1016/j.jtauto.2025.100280_bib108",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.jaut.2023.103070",
"article-title": "Molecular mimicry and autoimmunity in the time of COVID-19",
"author": "Rojas",
"doi-asserted-by": "crossref",
"journal-title": "J. Autoimmun.",
"key": "10.1016/j.jtauto.2025.100280_bib109",
"volume": "139",
"year": "2023"
},
{
"DOI": "10.1111/aji.13494",
"article-title": "Molecular mimicry between SARS-CoV-2 and the female reproductive system",
"author": "Dotan",
"doi-asserted-by": "crossref",
"journal-title": "Am. J. Reprod. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib110",
"volume": "86",
"year": "2021"
},
{
"DOI": "10.1007/s12026-020-09152-6",
"article-title": "Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine",
"author": "Kanduc",
"doi-asserted-by": "crossref",
"first-page": "310",
"journal-title": "Immunol. Res.",
"key": "10.1016/j.jtauto.2025.100280_bib111",
"volume": "68",
"year": "2020"
},
{
"DOI": "10.1111/bjh.16883",
"article-title": "Is molecular mimicry the culprit in the autoimmune haemolytic anaemia affecting patients with COVID-19?",
"author": "Angileri",
"doi-asserted-by": "crossref",
"first-page": "e92",
"journal-title": "Br. J. Haematol.",
"key": "10.1016/j.jtauto.2025.100280_bib112",
"volume": "190",
"year": "2020"
},
{
"DOI": "10.1016/j.clim.2020.108480",
"article-title": "Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases",
"author": "Vojdani",
"doi-asserted-by": "crossref",
"journal-title": "Clin. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib113",
"volume": "217",
"year": "2020"
},
{
"DOI": "10.1007/s12192-020-01145-6",
"article-title": "SARS-CoV-2 and Guillain-Barré syndrome: molecular mimicry with human heat shock proteins as potential pathogenic mechanism",
"author": "Lucchese",
"doi-asserted-by": "crossref",
"first-page": "731",
"journal-title": "Cell Stress Chaperones",
"key": "10.1016/j.jtauto.2025.100280_bib114",
"volume": "25",
"year": "2020"
},
{
"author": "Adiguzel",
"key": "10.1016/j.jtauto.2025.100280_bib115"
},
{
"author": "Adiguzel",
"first-page": "43",
"key": "10.1016/j.jtauto.2025.100280_bib116",
"series-title": "Chapter 4 - Molecular Mimicry Study between Peptides of SARS-CoV-2 and Neutrophil Extracellular Traps-Related Proteins",
"year": "2024"
},
{
"DOI": "10.3389/fimmu.2023.1267091",
"article-title": "The role of inflammation in autoimmune disease: a therapeutic target",
"author": "Xiang",
"doi-asserted-by": "crossref",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib117",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1093/bib/bbac056",
"article-title": "CRESSP: a comprehensive pipeline for prediction of immunopathogenic SARS-CoV-2 epitopes using structural properties of proteins",
"author": "An",
"doi-asserted-by": "crossref",
"journal-title": "Briefings Bioinf.",
"key": "10.1016/j.jtauto.2025.100280_bib118",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1007/s00281-016-0558-0",
"article-title": "The host immune response in respiratory virus infection: balancing virus clearance and immunopathology",
"author": "Newton",
"doi-asserted-by": "crossref",
"first-page": "471",
"journal-title": "Semin. Immunopathol.",
"key": "10.1016/j.jtauto.2025.100280_bib119",
"volume": "38",
"year": "2016"
},
{
"DOI": "10.1097/SHK.0000000000001740",
"article-title": "Immunopathological roles of neutrophils in virus infection and COVID-19",
"author": "Cui",
"doi-asserted-by": "crossref",
"first-page": "345",
"journal-title": "Shock",
"key": "10.1016/j.jtauto.2025.100280_bib120",
"volume": "56",
"year": "2021"
},
{
"DOI": "10.1080/21505594.2021.1898790",
"article-title": "Immune dysregulation and system pathology in COVID-19",
"author": "Jamal",
"doi-asserted-by": "crossref",
"first-page": "918",
"journal-title": "Virulence",
"key": "10.1016/j.jtauto.2025.100280_bib121",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1084/jem.20201129",
"article-title": "SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology",
"author": "Veras",
"doi-asserted-by": "crossref",
"journal-title": "J. Exp. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib122",
"volume": "217",
"year": "2020"
},
{
"DOI": "10.1111/imr.13175",
"article-title": "Neutrophils during SARS-CoV-2 infection: friend or foe?",
"author": "Castanheira",
"doi-asserted-by": "crossref",
"first-page": "399",
"journal-title": "Immunol. Rev.",
"key": "10.1016/j.jtauto.2025.100280_bib123",
"volume": "314",
"year": "2023"
},
{
"DOI": "10.3389/fmolb.2020.00157",
"article-title": "Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19",
"author": "Zhang",
"doi-asserted-by": "crossref",
"journal-title": "Front. Mol. Biosci.",
"key": "10.1016/j.jtauto.2025.100280_bib124",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Lancet",
"key": "10.1016/j.jtauto.2025.100280_bib125",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "10.1016/j.jtauto.2025.100280_bib126",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.02063",
"article-title": "Excessive neutrophils and neutrophil extracellular traps in COVID-19",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "2063",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib127",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1172/jci.insight.138999",
"article-title": "Neutrophil extracellular traps in COVID-19",
"author": "Zuo",
"doi-asserted-by": "crossref",
"journal-title": "JCI Insight",
"key": "10.1016/j.jtauto.2025.100280_bib128",
"year": "2020"
},
{
"DOI": "10.1186/s13073-020-00823-5",
"article-title": "Disease severity-specific neutrophil signatures in blood transcriptomes stratify COVID-19 patients",
"author": "Aschenbrenner",
"doi-asserted-by": "crossref",
"first-page": "7",
"journal-title": "Genome Med.",
"key": "10.1016/j.jtauto.2025.100280_bib129",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.ajpath.2017.09.014",
"article-title": "The role of extracellular histones in influenza virus pathogenesis",
"author": "Ashar",
"doi-asserted-by": "crossref",
"first-page": "135",
"journal-title": "Am. J. Pathol.",
"key": "10.1016/j.jtauto.2025.100280_bib130",
"volume": "188",
"year": "2018"
},
{
"DOI": "10.1186/s12931-023-02650-9",
"article-title": "What is the actual relationship between neutrophil extracellular traps and COVID-19 severity? A longitudinal study",
"author": "de Diego",
"doi-asserted-by": "crossref",
"first-page": "48",
"journal-title": "Respir. Res.",
"key": "10.1016/j.jtauto.2025.100280_bib131",
"volume": "25",
"year": "2024"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical characteristics of coronavirus disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib132",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/j.thromres.2020.04.013",
"article-title": "Incidence of thrombotic complications in critically ill ICU patients with COVID-19",
"author": "Klok",
"doi-asserted-by": "crossref",
"first-page": "145",
"journal-title": "Thromb. Res.",
"key": "10.1016/j.jtauto.2025.100280_bib133",
"volume": "191",
"year": "2020"
},
{
"DOI": "10.1177/1074248420958973",
"article-title": "COVID-19 infection: viral macro- and micro-vascular coagulopathy and thromboembolism/prophylactic and therapeutic management",
"author": "Manolis",
"doi-asserted-by": "crossref",
"first-page": "12",
"journal-title": "J. Cardiovasc. Pharmacol. Therapeut.",
"key": "10.1016/j.jtauto.2025.100280_bib134",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1182/blood.2020007008",
"article-title": "Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome",
"author": "Middleton",
"doi-asserted-by": "crossref",
"first-page": "1169",
"journal-title": "Blood",
"key": "10.1016/j.jtauto.2025.100280_bib135",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.1161/CIRCULATIONAHA.120.048488",
"article-title": "Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy",
"author": "Nicolai",
"doi-asserted-by": "crossref",
"first-page": "1176",
"journal-title": "Circulation",
"key": "10.1016/j.jtauto.2025.100280_bib136",
"volume": "142",
"year": "2020"
},
{
"DOI": "10.1038/s41418-021-00805-z",
"article-title": "Patients with COVID-19: in the dark-NETs of neutrophils",
"author": "Ackermann",
"doi-asserted-by": "crossref",
"first-page": "3125",
"journal-title": "Cell Death Differ.",
"key": "10.1016/j.jtauto.2025.100280_bib137",
"volume": "28",
"year": "2021"
},
{
"key": "10.1016/j.jtauto.2025.100280_bib138",
"series-title": "Post COVID-19 Condition (Long COVID)",
"year": "2022"
},
{
"DOI": "10.1001/jamainternmed.2023.0750",
"article-title": "Risk factors associated with post-COVID-19 condition: a systematic review and meta-analysis",
"author": "Tsampasian",
"doi-asserted-by": "crossref",
"first-page": "566",
"journal-title": "JAMA Intern. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib139",
"volume": "183",
"year": "2023"
},
{
"DOI": "10.1016/S2213-2600(21)00031-X",
"article-title": "NICE guideline on long COVID",
"author": "Venkatesan",
"doi-asserted-by": "crossref",
"first-page": "129",
"journal-title": "Lancet Respir. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib140",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.autrev.2021.102947",
"article-title": "Post-COVID syndrome. A case series and comprehensive review",
"author": "Anaya",
"doi-asserted-by": "crossref",
"journal-title": "Autoimmun. Rev.",
"key": "10.1016/j.jtauto.2025.100280_bib141",
"volume": "20",
"year": "2021"
},
{
"article-title": "Post-COVID syndrome: the aftershock of SARS-CoV-2",
"author": "Dotan",
"first-page": "233",
"journal-title": "Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis.",
"key": "10.1016/j.jtauto.2025.100280_bib142",
"volume": "114",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2021.101019",
"article-title": "Characterizing long COVID in an international cohort: 7 months of symptoms and their impact",
"author": "Davis",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "10.1016/j.jtauto.2025.100280_bib143",
"volume": "38",
"year": "2021"
},
{
"DOI": "10.15585/mmwr.mm7121e1",
"article-title": "Post–COVID conditions among adult COVID-19 survivors aged 18–64 and ≥65 Years — United States, march 2020–november 2021",
"author": "Bull-Otterson",
"doi-asserted-by": "crossref",
"first-page": "713",
"journal-title": "Morb. Mortal. Wkly. Rep.",
"key": "10.1016/j.jtauto.2025.100280_bib144",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2021.01.004",
"article-title": "Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study",
"author": "Moreno-Pérez",
"doi-asserted-by": "crossref",
"first-page": "378",
"journal-title": "J. Infect.",
"key": "10.1016/j.jtauto.2025.100280_bib145",
"volume": "82",
"year": "2021"
},
{
"DOI": "10.7554/eLife.86002",
"article-title": "Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC)",
"author": "Sherif",
"doi-asserted-by": "crossref",
"journal-title": "Elife",
"key": "10.1016/j.jtauto.2025.100280_bib146",
"volume": "12",
"year": "2023"
},
{
"key": "10.1016/j.jtauto.2025.100280_bib147",
"unstructured": "National Center for Health Statistics. U.S. Census Bureau, Household Pulse Survey, 2022–2023. Long COVID. Generated interactively: from https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm, (n.d.)."
},
{
"DOI": "10.1038/s41579-022-00846-2",
"article-title": "Long COVID: major findings, mechanisms and recommendations",
"author": "Davis",
"doi-asserted-by": "crossref",
"first-page": "133",
"journal-title": "Nat. Rev. Microbiol.",
"key": "10.1016/j.jtauto.2025.100280_bib148",
"volume": "21",
"year": "2023"
},
{
"article-title": "Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study",
"author": "Augustin",
"journal-title": "Lancet Reg. Heal. - Eur.",
"key": "10.1016/j.jtauto.2025.100280_bib149",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciac722",
"article-title": "Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae",
"author": "Swank",
"doi-asserted-by": "crossref",
"first-page": "e487",
"journal-title": "Clin. Infect. Dis. an Off. Publ. Infect. Dis. Soc. Am.",
"key": "10.1016/j.jtauto.2025.100280_bib150",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.3389/fmicb.2021.698169",
"article-title": "Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms",
"author": "Proal",
"doi-asserted-by": "crossref",
"journal-title": "Front. Microbiol.",
"key": "10.1016/j.jtauto.2025.100280_bib151",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1136/gutjnl-2021-325989",
"article-title": "Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "544",
"journal-title": "Gut",
"key": "10.1016/j.jtauto.2025.100280_bib152",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2022.01.014",
"article-title": "Multiple early factors anticipate post-acute COVID-19 sequelae",
"author": "Su",
"doi-asserted-by": "crossref",
"first-page": "881",
"journal-title": "Cell",
"key": "10.1016/j.jtauto.2025.100280_bib153",
"volume": "185",
"year": "2022"
},
{
"DOI": "10.1186/s12967-022-03346-2",
"article-title": "Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS)",
"author": "Haffke",
"doi-asserted-by": "crossref",
"first-page": "138",
"journal-title": "J. Transl. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib154",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1126/science.abm2052",
"article-title": "Nervous system consequences of COVID-19",
"author": "Spudich",
"doi-asserted-by": "crossref",
"first-page": "267",
"journal-title": "Science",
"key": "10.1016/j.jtauto.2025.100280_bib155",
"volume": "375",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiac017",
"article-title": "Persistent autoimmune activation and proinflammatory state in post-coronavirus disease 2019 syndrome",
"author": "Acosta-Ampudia",
"doi-asserted-by": "crossref",
"first-page": "2155",
"journal-title": "J. Infect. Dis.",
"key": "10.1016/j.jtauto.2025.100280_bib156",
"volume": "225",
"year": "2022"
},
{
"DOI": "10.1186/s12967-022-03328-4",
"article-title": "Autoimmunity is a hallmark of post-COVID syndrome",
"author": "Rojas",
"doi-asserted-by": "crossref",
"first-page": "129",
"journal-title": "J. Transl. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib157",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1016/j.jaut.2021.102682",
"article-title": "Unique autoantibody prevalence in long-term recovered SARS-CoV-2-infected individuals",
"author": "Lingel",
"doi-asserted-by": "crossref",
"journal-title": "J. Autoimmun.",
"key": "10.1016/j.jtauto.2025.100280_bib158",
"volume": "122",
"year": "2021"
},
{
"article-title": "Persistent IgG anticardiolipin autoantibodies are associated with post-COVID syndrome",
"author": "Bertin",
"first-page": "23",
"journal-title": "Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis.",
"key": "10.1016/j.jtauto.2025.100280_bib159",
"volume": "113",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciab611",
"article-title": "Persistent symptoms in adult patients 1 Year after coronavirus disease 2019 (COVID-19): a prospective cohort study",
"author": "Seeßle",
"doi-asserted-by": "crossref",
"first-page": "1191",
"journal-title": "Clin. Infect. Dis. an Off. Publ. Infect. Dis. Soc. Am.",
"key": "10.1016/j.jtauto.2025.100280_bib160",
"volume": "74",
"year": "2022"
},
{
"DOI": "10.1007/s10067-023-06670-0",
"article-title": "Incident autoimmune diseases in association with SARS-CoV-2 infection: a matched cohort study",
"author": "Tesch",
"doi-asserted-by": "crossref",
"first-page": "2905",
"journal-title": "Clin. Rheumatol.",
"key": "10.1016/j.jtauto.2025.100280_bib161",
"volume": "42",
"year": "2023"
},
{
"DOI": "10.1016/j.eclinm.2022.101783",
"article-title": "Risk of autoimmune diseases in patients with COVID-19: a retrospective cohort study",
"author": "Chang",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "10.1016/j.jtauto.2025.100280_bib162",
"volume": "56",
"year": "2023"
},
{
"DOI": "10.1016/j.autrev.2023.103409",
"article-title": "Increased incidence of rheumatoid arthritis after COVID-19",
"author": "Marín",
"doi-asserted-by": "crossref",
"journal-title": "Autoimmun. Rev.",
"key": "10.1016/j.jtauto.2025.100280_bib163",
"volume": "22",
"year": "2023"
},
{
"article-title": "Persistent risk of developing autoimmune diseases associated with COVID-19: an observational study using an electronic medical record database in Japan",
"author": "Inokuchi",
"first-page": "65",
"journal-title": "J. Clin. Rheumatol. Pract. Reports Rheum. Musculoskelet. Dis.",
"key": "10.1016/j.jtauto.2025.100280_bib164",
"volume": "30",
"year": "2024"
},
{
"article-title": "A causal link between autoantibodies and neurological symptoms in long COVID",
"author": "Santos Guedes de Sa",
"journal-title": "MedRxiv Prepr. Serv. Heal. Sci.",
"key": "10.1016/j.jtauto.2025.100280_bib165",
"year": "2024"
},
{
"DOI": "10.1002/jmv.28209",
"article-title": "Persistence of neutrophil extracellular traps and anticardiolipin auto-antibodies in post-acute phase COVID-19 patients",
"author": "Pisareva",
"doi-asserted-by": "crossref",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.jtauto.2025.100280_bib166",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1038/s41467-023-40012-7",
"article-title": "Chronic inflammation, neutrophil activity, and autoreactivity splits long COVID",
"author": "Woodruff",
"doi-asserted-by": "crossref",
"first-page": "4201",
"journal-title": "Nat. Commun.",
"key": "10.1016/j.jtauto.2025.100280_bib167",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1038/s41467-022-31897-x",
"article-title": "A nationwide questionnaire study of post-acute symptoms and health problems after SARS-CoV-2 infection in Denmark",
"author": "Sørensen",
"doi-asserted-by": "crossref",
"first-page": "4213",
"journal-title": "Nat. Commun.",
"key": "10.1016/j.jtauto.2025.100280_bib168",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3906/sag-2106-238",
"article-title": "Post-COVID syndrome: pulmonary complications",
"author": "Esendağli",
"doi-asserted-by": "crossref",
"first-page": "3359",
"journal-title": "Turk. J. Med. Sci.",
"key": "10.1016/j.jtauto.2025.100280_bib169",
"volume": "51",
"year": "2021"
},
{
"DOI": "10.1126/scitranslmed.abo5795",
"article-title": "A persistent neutrophil-associated immune signature characterizes post–COVID-19 pulmonary sequelae",
"author": "George",
"doi-asserted-by": "crossref",
"journal-title": "Sci. Transl. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib170",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1002/jmv.29887",
"article-title": "Inflammation-, immunothrombosis,- and autoimmune-feedback loops may lead to persistent neutrophil self-stimulation in long COVID",
"author": "Thierry",
"doi-asserted-by": "crossref",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.jtauto.2025.100280_bib171",
"volume": "96",
"year": "2024"
},
{
"DOI": "10.1016/j.imlet.2020.09.005",
"article-title": "How NETosis could drive “Post-COVID-19 syndrome” among survivors",
"author": "Sawadogo",
"doi-asserted-by": "crossref",
"first-page": "35",
"journal-title": "Immunol. Lett.",
"key": "10.1016/j.jtauto.2025.100280_bib172",
"volume": "228",
"year": "2020"
},
{
"DOI": "10.3389/fmed.2023.1083242",
"article-title": "Neutrophil extracellular traps in central nervous system pathologies: a mini review",
"author": "Shafqat",
"doi-asserted-by": "crossref",
"journal-title": "Front. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib173",
"volume": "10",
"year": "2023"
},
{
"article-title": "NETosis and neutrophil extracellular traps in COVID-19: immunothrombosis and beyond",
"author": "Zhu",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib174",
"volume": "13",
"year": "2022"
},
{
"article-title": "Role of NETosis in central nervous system injury",
"author": "Chen",
"journal-title": "Oxid. Med. Cell. Longev.",
"key": "10.1016/j.jtauto.2025.100280_bib175",
"volume": "2022",
"year": "2022"
},
{
"DOI": "10.3390/cells12050816",
"article-title": "Pathogenesis underlying neurological manifestations of long COVID syndrome and potential therapeutics",
"author": "Leng",
"doi-asserted-by": "crossref",
"journal-title": "Cells",
"key": "10.1016/j.jtauto.2025.100280_bib176",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.5607/en20048",
"article-title": "Neutrophil extracellular traps in coronavirus disease-19-associated ischemic stroke: a novel avenue in neuroscience",
"author": "Pramitasuri",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Exp. Neurobiol.",
"key": "10.1016/j.jtauto.2025.100280_bib177",
"volume": "30",
"year": "2021"
},
{
"DOI": "10.1177/00033197231153204",
"article-title": "Vascular “long COVID”: a new vessel disease?",
"author": "Zanini",
"doi-asserted-by": "crossref",
"first-page": "8",
"journal-title": "Angiology",
"key": "10.1016/j.jtauto.2025.100280_bib178",
"volume": "75",
"year": "2024"
},
{
"DOI": "10.1007/s00259-020-05084-3",
"article-title": "Vasculitis changes in COVID-19 survivors with persistent symptoms: an [(18)F]FDG-PET/CT study",
"author": "Sollini",
"doi-asserted-by": "crossref",
"first-page": "1460",
"journal-title": "Eur. J. Nucl. Med. Mol. Imag.",
"key": "10.1016/j.jtauto.2025.100280_bib179",
"volume": "48",
"year": "2021"
},
{
"DOI": "10.1038/s41591-022-01689-3",
"article-title": "Long-term cardiovascular outcomes of COVID-19",
"author": "Xie",
"doi-asserted-by": "crossref",
"first-page": "583",
"journal-title": "Nat. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib180",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1172/JCI141374",
"article-title": "Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis",
"author": "Skendros",
"doi-asserted-by": "crossref",
"first-page": "6151",
"journal-title": "J. Clin. Investig.",
"key": "10.1016/j.jtauto.2025.100280_bib181",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1007/s10875-023-01459-x",
"article-title": "Neutrophil activation and immune thrombosis profiles persist in convalescent COVID-19",
"author": "Hocini",
"doi-asserted-by": "crossref",
"first-page": "882",
"journal-title": "J. Clin. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib182",
"volume": "43",
"year": "2023"
},
{
"DOI": "10.1038/s41374-022-00783-x",
"article-title": "Characterization of COVID-19-associated cardiac injury: evidence for a multifactorial disease in an autopsy cohort",
"author": "Hanson",
"doi-asserted-by": "crossref",
"first-page": "814",
"journal-title": "Lab. Invest.",
"key": "10.1016/j.jtauto.2025.100280_bib183",
"volume": "102",
"year": "2022"
},
{
"DOI": "10.3390/jcm11092460",
"article-title": "Insights into the role of neutrophils and neutrophil extracellular traps in causing cardiovascular complications in patients with COVID-19: a systematic review",
"author": "Nappi",
"doi-asserted-by": "crossref",
"journal-title": "J. Clin. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib184",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/j.autrev.2021.102792",
"article-title": "The SARS-CoV-2 as an instrumental trigger of autoimmunity",
"author": "Dotan",
"doi-asserted-by": "crossref",
"journal-title": "Autoimmun. Rev.",
"key": "10.1016/j.jtauto.2025.100280_bib185",
"volume": "20",
"year": "2021"
},
{
"DOI": "10.1016/j.intimp.2022.109183",
"article-title": "Molecular mimicry, hyperactive immune system, and SARS-COV-2 are three prerequisites of the autoimmune disease triangle following COVID-19 infection",
"author": "Vahabi",
"doi-asserted-by": "crossref",
"journal-title": "Int. Immunopharmacol.",
"key": "10.1016/j.jtauto.2025.100280_bib186",
"volume": "112",
"year": "2022"
},
{
"DOI": "10.1126/scitranslmed.3001201",
"article-title": "Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus",
"author": "Garcia-Romo",
"doi-asserted-by": "crossref",
"journal-title": "Sci. Transl. Med.",
"key": "10.1016/j.jtauto.2025.100280_bib187",
"volume": "3",
"year": "2011"
},
{
"DOI": "10.1038/s41467-020-19412-6",
"article-title": "Type I IFN exacerbates disease in tuberculosis-susceptible mice by inducing neutrophil-mediated lung inflammation and NETosis",
"author": "Moreira-Teixeira",
"doi-asserted-by": "crossref",
"first-page": "5566",
"journal-title": "Nat. Commun.",
"key": "10.1016/j.jtauto.2025.100280_bib188",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1038/icb.2012.10",
"article-title": "Role of type I interferons in the activation of autoreactive B cells",
"author": "Kiefer",
"doi-asserted-by": "crossref",
"first-page": "498",
"journal-title": "Immunol. Cell Biol.",
"key": "10.1016/j.jtauto.2025.100280_bib189",
"volume": "90",
"year": "2012"
},
{
"DOI": "10.1016/j.celrep.2018.06.046",
"article-title": "B-Cell-Intrinsic type 1 interferon signaling is crucial for loss of tolerance and the development of autoreactive B cells",
"author": "Domeier",
"doi-asserted-by": "crossref",
"first-page": "406",
"journal-title": "Cell Rep.",
"key": "10.1016/j.jtauto.2025.100280_bib190",
"volume": "24",
"year": "2018"
},
{
"DOI": "10.1073/pnas.2003603117",
"article-title": "Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "16481",
"journal-title": "Proc. Natl. Acad. Sci. U.S.A.",
"key": "10.1016/j.jtauto.2025.100280_bib191",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2023.1254310",
"article-title": "Neutrophil extracellular traps and long COVID",
"author": "Shafqat",
"doi-asserted-by": "crossref",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib192",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.3390/cells10102545",
"article-title": "Neutrophil extracellular traps contribute to COVID-19 hyperinflammation and humoral autoimmunity",
"author": "Torres-Ruiz",
"doi-asserted-by": "crossref",
"journal-title": "Cells",
"key": "10.1016/j.jtauto.2025.100280_bib193",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03631-y",
"article-title": "Diverse functional autoantibodies in patients with COVID-19",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "283",
"journal-title": "Nature",
"key": "10.1016/j.jtauto.2025.100280_bib194",
"volume": "595",
"year": "2021"
},
{
"DOI": "10.1002/rmv.2412",
"article-title": "Partners in crime: autoantibodies complicit in COVID-19 pathogenesis",
"author": "Taghadosi",
"doi-asserted-by": "crossref",
"journal-title": "Rev. Med. Virol.",
"key": "10.1016/j.jtauto.2025.100280_bib195",
"volume": "33",
"year": "2023"
},
{
"DOI": "10.3389/fimmu.2021.680134",
"article-title": "Neutrophils and COVID-19: active participants and rational therapeutic targets",
"author": "Hazeldine",
"doi-asserted-by": "crossref",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.jtauto.2025.100280_bib196",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1186/s40560-014-0067-y",
"article-title": "Effect of a selective neutrophil elastase inhibitor on mortality and ventilator-free days in patients with increased extravascular lung water: a post hoc analysis of the PiCCO Pulmonary Edema Study",
"author": "Tagami",
"doi-asserted-by": "crossref",
"first-page": "67",
"journal-title": "J. Intensive Care.",
"key": "10.1016/j.jtauto.2025.100280_bib197",
"volume": "2",
"year": "2014"
},
{
"DOI": "10.3390/biomedicines9121867",
"article-title": "PAD inhibitors as a potential treatment for SARS-CoV-2 immunothrombosis",
"author": "Elliott",
"doi-asserted-by": "crossref",
"journal-title": "Biomedicines",
"key": "10.1016/j.jtauto.2025.100280_bib198",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1186/s13054-022-04062-5",
"article-title": "Gasdermin-D activation by SARS-CoV-2 triggers NET and mediate COVID-19 immunopathology",
"author": "Silva",
"doi-asserted-by": "crossref",
"first-page": "206",
"journal-title": "Crit. Care",
"key": "10.1016/j.jtauto.2025.100280_bib199",
"volume": "26",
"year": "2022"
},
{
"DOI": "10.1016/j.intimp.2021.108516",
"article-title": "Neutrophil Extracellular Traps (NETs) and Covid-19: a new frontiers for therapeutic modality",
"author": "Al-Kuraishy",
"doi-asserted-by": "crossref",
"journal-title": "Int. Immunopharmacol.",
"key": "10.1016/j.jtauto.2025.100280_bib200",
"volume": "104",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(23)00299-2",
"article-title": "Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial",
"author": "Bramante",
"doi-asserted-by": "crossref",
"first-page": "1119",
"journal-title": "Lancet Infect. Dis.",
"key": "10.1016/j.jtauto.2025.100280_bib201",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1007/s11239-022-02667-9",
"article-title": "Metformin therapy in COVID-19: inhibition of NETosis",
"author": "Kow",
"doi-asserted-by": "crossref",
"first-page": "217",
"journal-title": "J. Thromb. Thrombolysis",
"key": "10.1016/j.jtauto.2025.100280_bib202",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1007/s00592-018-1129-8",
"article-title": "The antidiabetic drug metformin blunts NETosis in vitro and reduces circulating NETosis biomarkers in vivo",
"author": "Menegazzo",
"doi-asserted-by": "crossref",
"first-page": "593",
"journal-title": "Acta Diabetol.",
"key": "10.1016/j.jtauto.2025.100280_bib203",
"volume": "55",
"year": "2018"
}
],
"reference-count": 203,
"references-count": 203,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2589909025000152"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "NETosis: A key player in autoimmunity, COVID-19, and long COVID",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy",
"volume": "10"
}