Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial
et al., The Lancet Infectious Diseases, doi:10.1016/S1473-3099(23)00299-2, NCT04510194, Dec 2022
Metformin for COVID-19
3rd treatment shown to reduce risk in
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
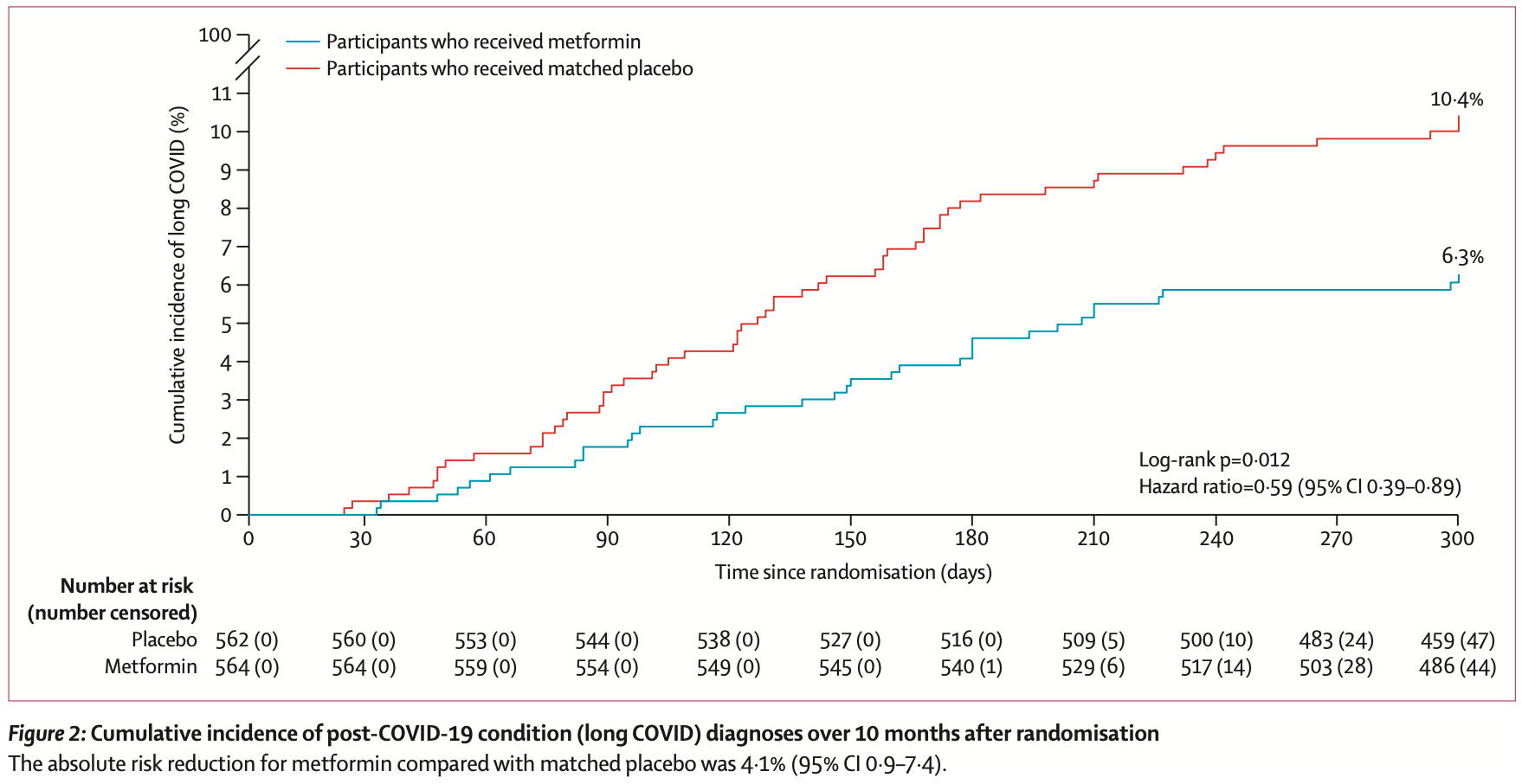
Long-term 10 month followup for NCT04510194 (history), showing significantly lower incidence of PASC with metformin treatment. Adjusted results are provided for metformin but not for ivermectin or fluvoxamine. For many issues with this trial, see1.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments2.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
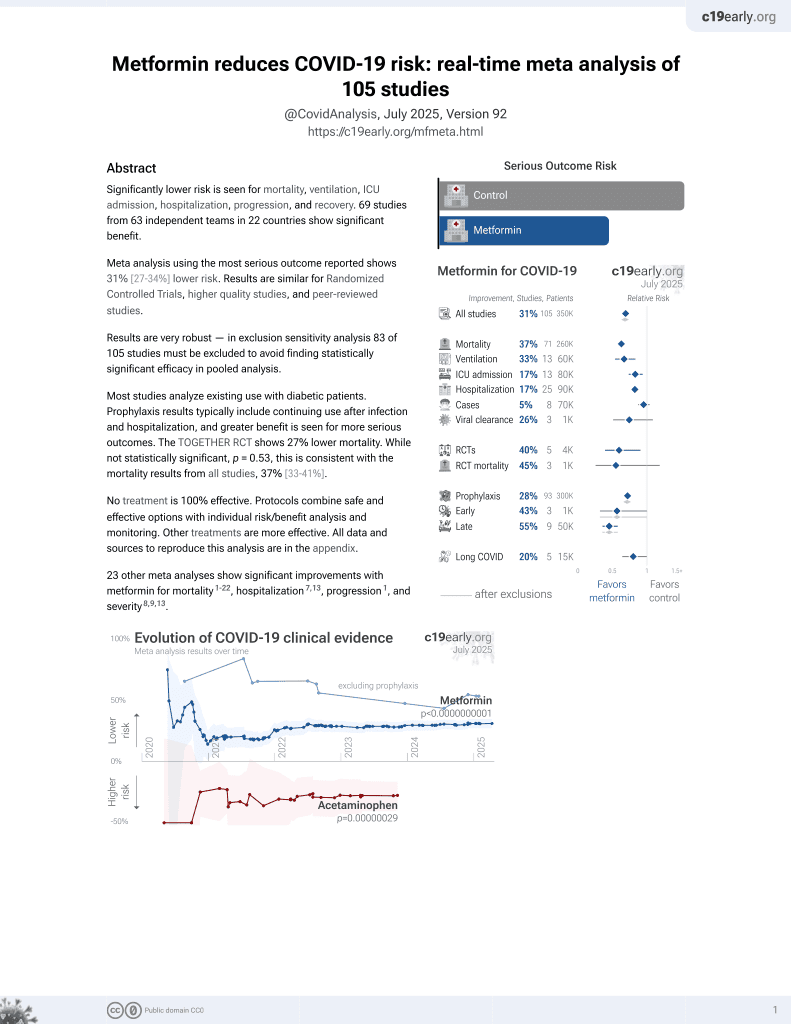
risk of long COVID, 41.2% lower, HR 0.59, p = 0.01, adjusted per study, metformin.
|
|
risk of long COVID, 1.4% lower, HR 0.99, p = 0.96, unadjusted, ivermectin.
|
|
risk of long COVID, 36.0% higher, HR 1.36, p = 0.28, unadjusted, fluvoxamine.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bramante et al., 24 Dec 2022, Randomized Controlled Trial, USA, peer-reviewed, median age 45.0, 31 authors, trial NCT04510194 (history).
Contact: bramante@umn.edu.
Outpatient treatment of Covid-19 with metformin, ivermectin, and fluvoxamine and the development of Long Covid over 10-month follow-up
doi:10.1101/2022.12.21.22283753
Background Long Covid is an emerging chronic illness potentially affecting millions, sometimes preventing the ability to work or participate in normal daily activities. COVID-OUT was an investigatorinitiated, multi-site, phase 3, randomized, quadruple-blinded placebo-controlled clinical trial (NCT04510194). The design simultaneously assessed three oral medications (metformin, ivermectin, fluvoxamine) using two by three parallel treatment factorial assignment to efficiently share placebo controls and assessed Long Covid outcomes for 10 months to understand whether early outpatient treatment of SARS-CoV-2 with metformin, ivermectin, or fluvoxamine prevents Long Covid. Methods: This was a decentralized, remotely delivered trial in the US of 1,125 adults age 30 to 85 with overweight or obesity, fewer than 7 days of symptoms, and enrolled within three days of a documented SARS-CoV-2 infection. Immediate release metformin titrated over 6 days to 1,500mg per day 14 days total; ivermectin 430mcg/kg/day for 3 days; fluvoxamine, 50mg on day one then 50mg twice daily through 14 days. Medical-provider diagnosis of Long Covid, reported by participant by day 300 after randomization was a pre-specified secondary outcome; the primary outcome of the trial was severe Covid by day 14.
Result: The median age was 45 years (IQR 37 to 54), 56% female of whom 7% were pregnant. Two percent identified as Native American; 3.7% as Asian; 7.4% as Black/African American; 82.8% as white; and 12.7% as Hispanic/Latino. The median BMI was 29.8 kg/m 2 (IQR 27 to 34); 51% had a BMI >30kg/m 2 . Overall, 8.4% reported having received a diagnosis of Long Covid from a medical provider: 6.3% in the metformin group and 10.6% in the metformin control; 8.0% in the ivermectin group and 8.1% in the ivermectin control; and 10.1% in the fluvoxamine group and 7.5% in the fluvoxamine control. The Hazard Ratio (HR) for Long Covid in the metformin group versus control was 0.58 (95% CI 0.38 to 0.88); 0.99 (95% CI 0.592 to 1.643) in the ivermectin group; and 1.36 in the fluvoxamine group (95% CI 0.785 to 2.385). Conclusions: There was a 42% relative decrease in the incidence of Long Covid in the metformin group compared to its blinded control in a secondary outcome of this randomized phase 3 trial.
Disclosures JBB reports contracted fees and travel support for contracted activities for consulting work paid to the University of North Carolina by Novo Nordisk; grant support by Dexcom, NovaTarg, Novo Nordisk, Sanofi, Tolerion and vTv Therapeutics; personal compensation for consultation from Alkahest, Altimmune, Anji, AstraZeneca, Bayer, Biomea Fusion Inc, Boehringer-Ingelheim, CeQur, Cirius Therapeutics Inc, Corcept Therapeutics, Eli Lilly, Fortress Biotech, GentiBio, Glycadia, Glyscend, Janssen, MannKind, Mellitus Health, Moderna, Pendulum Therapeutics, Praetego, Sanofi, Stability Health, Terns Inc, Valo and Zealand Pharma; and stock/options in Glyscend, Mellitus Health, Pendulum Therapeutics, PhaseBio, Praetego, and Stability Health.
References
Ballering, Van Zon, Hartman, Rosmalen, Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study, Lancet
Barnett, Sax, Long-term Follow-up After Critical COVID-19: REMAP-CAP Revisited, JAMA
Bramante, Huling, Tignanelli, Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19, New England Journal of Medicine
Brand, Saarelainen, Sonajalg, Metformin in pregnancy and risk of adverse long-term outcomes: a register-based cohort study, BMJ Open Diabetes Res Care
Castle, Dock, Hemmat, Biophysical modeling of the SARS-CoV-2 viral cycle reveals ideal antiviral targets, bioRxiv
Chang, Umpierrez, Inzucchi, Management of Hyperglycemia in Hospitalized, Non-Critically Ill Adults, The New England journal of medicine
Chen, Han, Yang, SARS-CoV-2 Infection Causes Dopaminergic Neuron Senescence, Res Sq
Cutler, The Costs of Long COVID, JAMA Health Forum
Declercq, Van Damme, Leeuw, Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): a factorial, randomised, controlled trial, Lancet Respir Med
Gordon, Jang, Bouhaddou, A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature
Haltmeier, Benjamin, Beale, Inaba, Demetriades, Insulin-Treated Patients with Diabetes Mellitus Undergoing Emergency Abdominal Surgery Have Worse Outcomes than Patients Treated with Oral Agents, World J Surg
Hosey, Halpin, Yan, Considering metformin as a second-line treatment for children and adolescents with prediabetes, Journal of pediatric endocrinology & metabolism
Hyer, Balani, Shehata, Metformin in Pregnancy: Mechanisms and Clinical Applications, Int J Mol Sci
Inzucchi, Lipska, Mayo, Bailey, Mcguire, Metformin in patients with type 2 diabetes and kidney disease: a systematic review, JAMA
Kalafat, Sukur, Abdi, Thilaganathan, Khalil, Metformin for prevention of hypertensive disorders of pregnancy in women with gestational diabetes or obesity: systematic review and meta-analysis of randomized trials, Ultrasound Obstet Gynecol
Karam, Morris, Bramante, mTOR inhibition in COVID-19: A commentary and review of efficacy in RNA viruses, Journal of medical virology
Manson, Cook, Lee, Marine n−3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer, New England Journal of Medicine
Manson, Cook, Lee, Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease, New England Journal of Medicine
Mantovani, Morrone, Patrono, Long Covid: where we stand and challenges ahead, Cell Death Differ
Masarwa, Brunetti, Aloe, Henderson, Platt et al., Efficacy and Safety of Metformin for Obesity: A Systematic Review, Pediatrics
Mcgrath, Scott, Surinach, Chambers, Benigno et al., Use of the Postacute Sequelae of COVID-19 Diagnosis Code in Routine Clinical Practice in the US, JAMA network open
Molloy, White, Nunn, Hayes, Wang et al., Multiplicity adjustments in parallel-group multi-arm trials sharing a control group: Clear guidance is needed, Contemp Clin Trials
Parker, Weir, Non-adjustment for multiple testing in multi-arm trials of distinct treatments: Rationale and justification, Clin Trials
Parthasarathy, Tandel, Siddiqui, Harshan, Metformin suppresses SARS-CoV-2 in cell culture, Virus research
Pasquel, Umpierrez, Web Exclusive. Annals for Hospitalists Inpatient Notes -How We Treat Hyperglycemia in the Hospital, Annals of internal medicine
Perlis, Santillana, Ognyanova, Prevalence and Correlates of Long COVID Symptoms Among US Adults, JAMA Netw Open
Pfaff, Girvin, Bennett, Identifying who has long COVID in the USA: a machine learning approach using N3C data, Lancet Digit Health
Pfaff, Girvin, Bennett, Identifying who has long COVID in the USA: a machine learning approach using N3C data, The Lancet Digital Health
Pocock, Stone, The Primary Outcome Fails -What Next? New England, Journal of Medicine
Rando, Bennett, Byrd, Challenges in defining Long COVID: Striking differences across literature, Electronic Health Records, and patient-reported information, medRxiv, doi:10.1101/2021.1103.1120.21253896
Reitz, Marroquin, Zenati, Association Between Preoperative Metformin Exposure and Postoperative Outcomes in Adults With Type 2 Diabetes, JAMA Surg
Salpeter, Greyber, Pasternak, Salpeter, Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus, Cochrane Database Syst Rev
Schaller, Sharma, Dupee, Ex vivo SARS-CoV-2 infection of human lung reveals heterogeneous host defense and therapeutic responses, JCI Insight
Skipper, Pastick, Engen, Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19 : A Randomized Trial, Annals of internal medicine
Sun, Liu, Huang, SARS-CoV-2 non-structural protein 6 triggers NLRP3-dependent pyroptosis by targeting ATP6AP1, Cell Death Differ
Ventura-López, Cervantes-Luevano, Aguirre-Sánchez, Treatment with metformin glycinate reduces SARS-CoV-2 viral load: An in vitro model and randomized, double-blind, Phase IIb clinical trial, Biomed Pharmacother
Warnakulasuriya, Fernando, Adikaram, Metformin in the Management of Childhood Obesity: A Randomized Control Trial, Child Obes
Yang, Zhao, Tebbutt, A glimpse into long COVID and symptoms, The Lancet Respiratory medicine
DOI record:
{
"DOI": "10.1016/s1473-3099(23)00299-2",
"ISSN": [
"1473-3099"
],
"URL": "http://dx.doi.org/10.1016/S1473-3099(23)00299-2",
"alternative-id": [
"S1473309923002992"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S1473-3099(23)00299-2"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S1473-3099(23)00355-9"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Bramante",
"given": "Carolyn T",
"sequence": "first"
},
{
"affiliation": [],
"family": "Buse",
"given": "John B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liebovitz",
"given": "David M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nicklas",
"given": "Jacinda M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Puskarich",
"given": "Michael A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cohen",
"given": "Ken",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Belani",
"given": "Hrishikesh K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anderson",
"given": "Blake J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huling",
"given": "Jared D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tignanelli",
"given": "Christopher J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thompson",
"given": "Jennifer L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pullen",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wirtz",
"given": "Esteban Lemus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Siegel",
"given": "Lianne K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Proper",
"given": "Jennifer L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Odde",
"given": "David J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Klatt",
"given": "Nichole R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sherwood",
"given": "Nancy E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lindberg",
"given": "Sarah M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Karger",
"given": "Amy B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beckman",
"given": "Kenneth B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Erickson",
"given": "Spencer M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fenno",
"given": "Sarah L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hartman",
"given": "Katrina M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rose",
"given": "Michael R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mehta",
"given": "Tanvi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Barkha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Griffiths",
"given": "Gwendolyn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhat",
"given": "Neeta S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murray",
"given": "Thomas A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boulware",
"given": "David R",
"sequence": "additional"
}
],
"container-title": "The Lancet Infectious Diseases",
"container-title-short": "The Lancet Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"em-consulte.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
6,
8
]
],
"date-time": "2023-06-08T22:33:22Z",
"timestamp": 1686263602000
},
"deposited": {
"date-parts": [
[
2023,
6,
8
]
],
"date-time": "2023-06-08T22:33:41Z",
"timestamp": 1686263621000
},
"indexed": {
"date-parts": [
[
2023,
6,
9
]
],
"date-time": "2023-06-09T04:38:25Z",
"timestamp": 1686285505129
},
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2023,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309923002992?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309923002992?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
6
]
]
},
"published-print": {
"date-parts": [
[
2023,
6
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/S2213-2600(22)00217-X",
"article-title": "A glimpse into long COVID and symptoms",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "e81",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S1473-3099(23)00299-2_bib2",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1001/jamahealthforum.2022.1809",
"article-title": "The costs of long COVID",
"author": "Cutler",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Health Forum",
"key": "10.1016/S1473-3099(23)00299-2_bib3",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1016/S2589-7500(22)00048-6",
"article-title": "Identifying who has long COVID in the USA: a machine learning approach using N3C data",
"author": "Pfaff",
"doi-asserted-by": "crossref",
"first-page": "e532",
"journal-title": "Lancet Digit Health",
"key": "10.1016/S1473-3099(23)00299-2_bib5",
"volume": "4",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2201662",
"article-title": "Randomized trial of metformin, ivermectin, and fluvoxamine for COVID-19",
"author": "Bramante",
"doi-asserted-by": "crossref",
"first-page": "599",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(23)00299-2_bib6",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1016/j.ajogmf.2022.100700",
"article-title": "Inclusion of pregnant and breastfeeding women in nonobstetrical randomized controlled trials",
"author": "Jorgensen",
"doi-asserted-by": "crossref",
"journal-title": "Am J Obstet Gynecol MFM",
"key": "10.1016/S1473-3099(23)00299-2_bib7",
"volume": "4",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)02467-9",
"article-title": "Pregnancy outcomes and vaccine effectiveness during the period of omicron as the variant of concern, INTERCOVID-2022: a multinational, observational study",
"author": "Villar",
"doi-asserted-by": "crossref",
"first-page": "447",
"journal-title": "Lancet",
"key": "10.1016/S1473-3099(23)00299-2_bib8",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.1002/uog.19084",
"article-title": "Metformin for prevention of hypertensive disorders of pregnancy in women with gestational diabetes or obesity: systematic review and meta-analysis of randomized trials",
"author": "Kalafat",
"doi-asserted-by": "crossref",
"first-page": "706",
"journal-title": "Ultrasound Obstet Gynecol",
"key": "10.1016/S1473-3099(23)00299-2_bib9",
"volume": "52",
"year": "2018"
},
{
"DOI": "10.1016/j.cct.2015.06.008",
"article-title": "Mass weighted urn design—a new randomization algorithm for unequal allocations",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "209",
"journal-title": "Contemp Clin Trials",
"key": "10.1016/S1473-3099(23)00299-2_bib10",
"volume": "43",
"year": "2015"
},
{
"DOI": "10.1186/1472-6947-12-127",
"article-title": "Healthcare provider attitudes towards the problem list in an electronic health record: a mixed-methods qualitative study",
"author": "Holmes",
"doi-asserted-by": "crossref",
"first-page": "127",
"journal-title": "BMC Med Inform Decis Mak",
"key": "10.1016/S1473-3099(23)00299-2_bib11",
"volume": "12",
"year": "2012"
},
{
"DOI": "10.1016/j.cct.2021.106656",
"article-title": "Multiplicity adjustments in parallel-group multi-arm trials sharing a control group: clear guidance is needed",
"author": "Molloy",
"doi-asserted-by": "crossref",
"journal-title": "Contemp Clin Trials",
"key": "10.1016/S1473-3099(23)00299-2_bib12",
"volume": "113",
"year": "2022"
},
{
"DOI": "10.1177/1740774520941419",
"article-title": "Non-adjustment for multiple testing in multi-arm trials of distinct treatments: rationale and justification",
"author": "Parker",
"doi-asserted-by": "crossref",
"first-page": "562",
"journal-title": "Clin Trials",
"key": "10.1016/S1473-3099(23)00299-2_bib13",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa1809944",
"article-title": "Vitamin D supplements and prevention of cancer and cardiovascular disease",
"author": "Manson",
"doi-asserted-by": "crossref",
"first-page": "33",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(23)00299-2_bib14",
"volume": "380",
"year": "2019"
},
{
"DOI": "10.1056/NEJMoa1811403",
"article-title": "Marine n-3 fatty acids and prevention of cardiovascular disease and cancer",
"author": "Manson",
"doi-asserted-by": "crossref",
"first-page": "23",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(23)00299-2_bib15",
"volume": "380",
"year": "2019"
},
{
"DOI": "10.1016/S2213-2600(21)00377-5",
"article-title": "Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): a factorial, randomised, controlled trial",
"author": "Declercq",
"doi-asserted-by": "crossref",
"first-page": "1427",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S1473-3099(23)00299-2_bib16",
"volume": "9",
"year": "2021"
},
{
"article-title": "Sparse data bias: a problem hiding in plain sight",
"author": "Greenland",
"journal-title": "BMJ",
"key": "10.1016/S1473-3099(23)00299-2_bib17",
"volume": "352",
"year": "2016"
},
{
"DOI": "10.1056/NEJMra1510064",
"article-title": "The primary outcome fails—what next?",
"author": "Pocock",
"doi-asserted-by": "crossref",
"first-page": "861",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(23)00299-2_bib18",
"volume": "375",
"year": "2016"
},
{
"DOI": "10.1016/S1473-3099(21)00703-9",
"article-title": "A clinical case definition of post-COVID-19 condition by a Delphi consensus",
"author": "Soriano",
"doi-asserted-by": "crossref",
"first-page": "e102",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S1473-3099(23)00299-2_bib20",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/S2214-109X(21)00448-4",
"article-title": "Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "e42",
"journal-title": "Lancet Glob Health",
"key": "10.1016/S1473-3099(23)00299-2_bib21",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.biopha.2022.113223",
"article-title": "Treatment with metformin glycinate reduces SARS-CoV-2 viral load: an in vitro model and randomized, double-blind, phase IIb clinical trial",
"author": "Ventura-López",
"doi-asserted-by": "crossref",
"journal-title": "Biomed Pharmacother",
"key": "10.1016/S1473-3099(23)00299-2_bib22",
"volume": "152",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2021.101019",
"article-title": "Characterizing long COVID in an international cohort: 7 months of symptoms and their impact",
"author": "Davis",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "10.1016/S1473-3099(23)00299-2_bib23",
"volume": "38",
"year": "2021"
},
{
"article-title": "Long COVID: where we stand and challenges ahead",
"author": "Mantovani",
"first-page": "1891",
"journal-title": "Cell Death Differ",
"key": "10.1016/S1473-3099(23)00299-2_bib24",
"volume": "29",
"year": "2022"
},
{
"article-title": "Biophysical modeling of the SARS-CoV-2 viral cycle reveals ideal antiviral targets",
"author": "Castle",
"journal-title": "bioRxiv",
"key": "10.1016/S1473-3099(23)00299-2_bib25",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26728",
"article-title": "mTOR inhibition in COVID-19: a commentary and review of efficacy in RNA viruses",
"author": "Karam",
"doi-asserted-by": "crossref",
"first-page": "1843",
"journal-title": "J Med Virol",
"key": "10.1016/S1473-3099(23)00299-2_bib26",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"article-title": "A SARS-CoV-2 protein interaction map reveals targets for drug repurposing",
"author": "Gordon",
"doi-asserted-by": "crossref",
"first-page": "459",
"journal-title": "Nature",
"key": "10.1016/S1473-3099(23)00299-2_bib27",
"volume": "583",
"year": "2020"
},
{
"article-title": "Metformin suppresses SARS-CoV-2 in cell culture",
"author": "Parthasarathy",
"journal-title": "Virus Res",
"key": "10.1016/S1473-3099(23)00299-2_bib28",
"volume": "323",
"year": "2022"
},
{
"DOI": "10.1172/jci.insight.148003",
"article-title": "Ex vivo SARS-CoV-2 infection of human lung reveals heterogeneous host defense and therapeutic responses",
"author": "Schaller",
"doi-asserted-by": "crossref",
"journal-title": "JCI Insight",
"key": "10.1016/S1473-3099(23)00299-2_bib29",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.3892/etm.2021.10010",
"article-title": "Metformin attenuates diabetic renal injury via the AMPK-autophagy axis",
"author": "Sun",
"doi-asserted-by": "crossref",
"first-page": "578",
"journal-title": "Exp Ther Med",
"key": "10.1016/S1473-3099(23)00299-2_bib30",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1038/s41418-021-00916-7",
"article-title": "SARS-CoV-2 non-structural protein 6 triggers NLRP3-dependent pyroptosis by targeting ATP6AP1",
"author": "Sun",
"doi-asserted-by": "crossref",
"first-page": "1240",
"journal-title": "Cell Death Differ",
"key": "10.1016/S1473-3099(23)00299-2_bib31",
"volume": "29",
"year": "2022"
},
{
"author": "Chen",
"key": "10.1016/S1473-3099(23)00299-2_bib32"
},
{
"DOI": "10.1002/14651858.CD002967.pub3",
"article-title": "Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus",
"author": "Salpeter",
"doi-asserted-by": "crossref",
"journal-title": "Cochrane Database Syst Rev",
"key": "10.1016/S1473-3099(23)00299-2_bib33",
"volume": "2010",
"year": "2010"
},
{
"DOI": "10.1161/CIRCHEARTFAILURE.112.000162",
"article-title": "Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients",
"author": "Eurich",
"doi-asserted-by": "crossref",
"first-page": "395",
"journal-title": "Circ Heart Fail",
"key": "10.1016/S1473-3099(23)00299-2_bib34",
"volume": "6",
"year": "2013"
},
{
"DOI": "10.1111/dom.14313",
"article-title": "Cardiovascular and renal safety of metformin in patients with diabetes and moderate or severe chronic kidney disease: observations from the EXSCEL and SAVOR-TIMI 53 cardiovascular outcomes trials",
"author": "Clegg",
"doi-asserted-by": "crossref",
"first-page": "1101",
"journal-title": "Diabetes Obes Metab",
"key": "10.1016/S1473-3099(23)00299-2_bib35",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1089/chi.2018.0043",
"article-title": "Metformin in the management of childhood obesity: a randomized control trial",
"author": "Warnakulasuriya",
"doi-asserted-by": "crossref",
"first-page": "553",
"journal-title": "Child Obes",
"key": "10.1016/S1473-3099(23)00299-2_bib36",
"volume": "14",
"year": "2018"
},
{
"DOI": "10.1056/NEJMclde2204691",
"article-title": "Management of hyperglycemia in hospitalized, non-critically ill adults",
"author": "Chang",
"doi-asserted-by": "crossref",
"first-page": "1040",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(23)00299-2_bib37",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1001/jamasurg.2020.0416",
"article-title": "Association between preoperative metformin exposure and postoperative outcomes in adults with type 2 diabetes",
"author": "Reitz",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Surg",
"key": "10.1016/S1473-3099(23)00299-2_bib38",
"volume": "155",
"year": "2020"
},
{
"DOI": "10.1016/S1470-2045(19)30854-X",
"article-title": "Statistical significance and clinical evidence",
"author": "Gates",
"doi-asserted-by": "crossref",
"first-page": "e118",
"journal-title": "Lancet Oncol",
"key": "10.1016/S1473-3099(23)00299-2_bib39",
"volume": "21",
"year": "2020"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1473309923002992"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases"
],
"subtitle": [],
"title": "Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}