Metformin is a potential therapeutic for COVID-19/LUAD by regulating glucose metabolism
et al., Scientific Reports, doi:10.1038/s41598-024-63081-0, May 2024
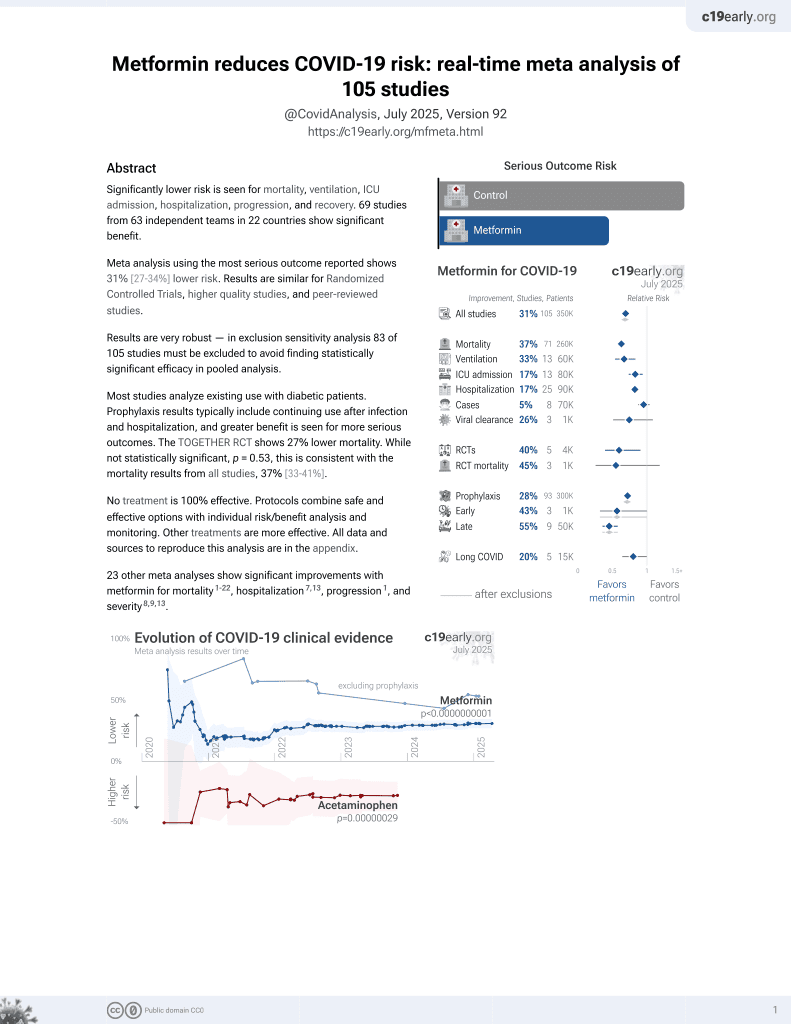
Metformin for COVID-19
3rd treatment shown to reduce risk in
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
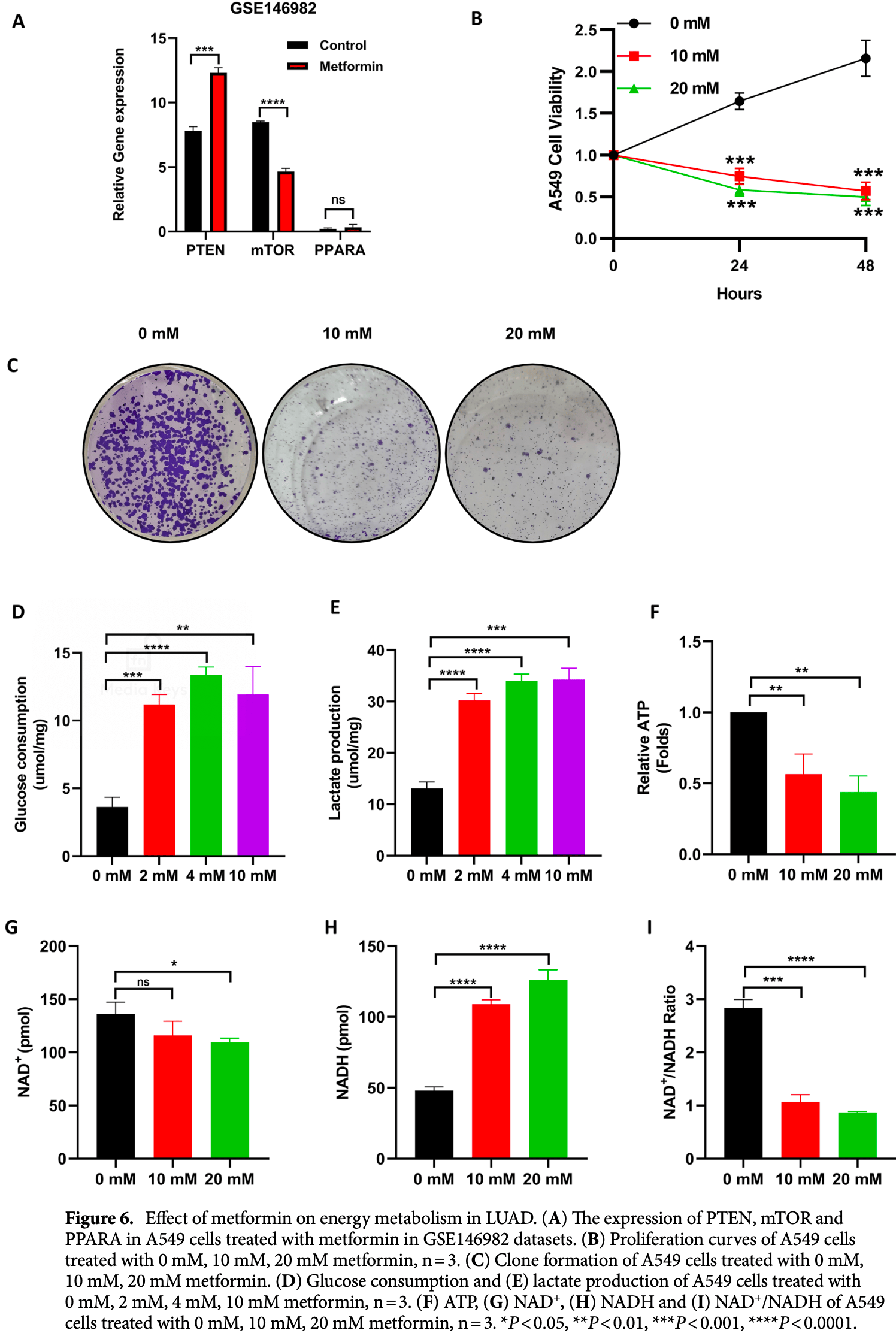
In silico and in vitro study showing metformin as a potential therapeutic for COVID-19/LUAD by regulating glucose metabolism. Authors identified PTEN and mTOR as potential core target genes of metformin for treating COVID-19/LUAD. Bioinformatics analysis suggests metformin's mechanism may involve energy metabolism, NADH oxidoreductase activity, FoxO signaling, AMPK signaling, and mTOR signaling pathways. In A549 lung adenocarcinoma cells, metformin increased PTEN expression, decreased mTOR expression, inhibited cell proliferation and colony formation, increased glucose consumption and lactate production, and decreased ATP production and NAD+/NADH ratio.
18 preclinical studies support the efficacy of metformin for COVID-19:
A systematic review and meta-analysis of 15 non-COVID-19 preclinical studies showed that metformin inhibits pulmonary inflammation and oxidative stress, minimizes lung injury, and improves survival in animal models of acute respiratory distress syndrome (ARDS) or acute lung injury (ALI)15.
Metformin inhibits SARS-CoV-2 in vitro11,12, minimizes LPS-induced cytokine storm in a mouse model14, minimizes lung damage and fibrosis in a mouse model of LPS-induced ARDS10, may protect against SARS-CoV-2-induced neurological disorders9, may be beneficial via inhibitory effects on ORF3a-mediated inflammasome activation16, reduces UUO and FAN-induced kidney fibrosis10, increases mitochondrial function and decreases TGF-β-induced fibrosis, apoptosis, and inflammation markers in lung epithelial cells10, may reduce inflammation, oxidative stress, and thrombosis via regulating glucose metabolism2, attenuates spike protein S1-induced inflammatory response and α-synuclein aggregation8, may protect against COVID-19 cognitive impairment by suppressing HIF-1α stabilization and reducing neurodegenerative protein aggregation13, may reduce COVID-19 severity and long COVID by inhibiting NETosis via suppression of protein kinase C activation17, enhances interferon responses and reduces SARS-CoV-2 infection and inflammation in diabetic models by suppressing HIF-1α signaling7, may improve COVID-19 outcomes by preventing VDAC1 mistargeting to the plasma membrane, reducing ATP loss, and preserving immune cell function during cytokine storm18, reduces hyperglycemia-induced hepatic ACE2/TMPRSS2 up-regulation and SARS-CoV-2 entry6, may reduce COVID-19 severity by suppressing monocyte inflammatory responses and glycolytic activation via AMPK pathway modulation5, and may improve outcomes via modulation of immune responses with increased anti-inflammatory T lymphocyte gene expression and via enhanced gut microbiota diversity19.
1.
Tavares et al., Investigation of Interactions Between the Protein MPro and the Vanadium Complex VO(metf)2∙H2O: A Computational Approach for COVID-19 Treatment, Biophysica, doi:10.3390/biophysica5010004.
2.
Hou et al., Metformin is a potential therapeutic for COVID-19/LUAD by regulating glucose metabolism, Scientific Reports, doi:10.1038/s41598-024-63081-0.
3.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
4.
Lockwood, T., Coordination chemistry suggests that independently observed benefits of metformin and Zn2+ against COVID-19 are not independent, BioMetals, doi:10.1007/s10534-024-00590-5.
5.
Maurmann et al., Immunoregulatory effect of metformin in monocytes exposed to SARS-CoV-2 spike protein subunit 1, bioRxiv, doi:10.1101/2025.09.12.675877.
6.
Rao et al., Pathological Glucose Levels Enhance Entry Factor Expression and Hepatic SARS‐CoV‐2 Infection, Journal of Cellular and Molecular Medicine, doi:10.1111/jcmm.70581.
7.
Joshi et al., Severe SARS‐CoV‐2 infection in diabetes was rescued in mice supplemented with metformin and/or αKG, and patients taking metformin, via HIF1α‐IFN axis, Clinical and Translational Medicine, doi:10.1002/ctm2.70275.
8.
Chang et al., SARS-CoV-2 Spike Protein 1 Causes Aggregation of α-Synuclein via Microglia-Induced Inflammation and Production of Mitochondrial ROS: Potential Therapeutic Applications of Metformin, Biomedicines, doi:10.3390/biomedicines12061223.
9.
Yang et al., SARS-CoV-2 infection causes dopaminergic neuron senescence, Cell Stem Cell, doi:10.1016/j.stem.2023.12.012.
10.
Miguel et al., Enhanced fatty acid oxidation through metformin and baicalin as therapy for COVID-19 and associated inflammatory states in lung and kidney, Redox Biology, doi:10.1016/j.redox.2023.102957.
11.
Ventura-López et al., Treatment with metformin glycinate reduces SARS-CoV-2 viral load: An in vitro model and randomized, double-blind, Phase IIb clinical trial, Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2022.113223.
12.
Parthasarathy et al., Metformin Suppresses SARS-CoV-2 in Cell Culture, bioRxiv, doi:10.1101/2021.11.18.469078.
13.
Lee et al., SARS-CoV-2 spike protein causes synaptic dysfunction and p-tau and α-synuclein aggregation leading cognitive impairment: The protective role of metformin, PLOS One, doi:10.1371/journal.pone.0336015.
14.
Taher et al., Anti‑inflammatory effect of metformin against an experimental model of LPS‑induced cytokine storm, Experimental and Therapeutic Medicine, doi:10.3892/etm.2023.12114.
15.
Wang et al., Effects of metformin on acute respiratory distress syndrome in preclinical studies: a systematic review and meta-analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2023.1215307.
16.
Zhang et al., SARS-CoV-2 ORF3a Protein as a Therapeutic Target against COVID-19 and Long-Term Post-Infection Effects, Pathogens, doi:10.3390/pathogens13010075.
17.
Monsalve et al., NETosis: A key player in autoimmunity, COVID-19, and long COVID, Journal of Translational Autoimmunity, doi:10.1016/j.jtauto.2025.100280.
Hou et al., 30 May 2024, peer-reviewed, 9 authors.
Contact: 408931519@qq.com, zbzb612@126.com.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Metformin is a potential therapeutic for COVID-19/LUAD by regulating glucose metabolism
Scientific Reports, doi:10.1038/s41598-024-63081-0
Lung adenocarcinoma (LUAD) is the most common and aggressive subtype of lung cancer, and coronavirus disease 2019 (COVID-19) has become a serious public health threat worldwide. Patients with LUAD and COVID-19 have a poor prognosis. Therefore, finding medications that can be used to treat COVID-19/LUAD patients is essential. Bioinformatics analysis was used to identify 20 possible metformin target genes for the treatment of COVID-19/LUAD. PTEN and mTOR may serve as hub target genes of metformin. Metformin may be able to cure COVID-19/LUAD comorbidity through energy metabolism, oxidoreductase NADH activity, FoxO signalling pathway, AMPK signalling system, and mTOR signalling pathway, among other pathways, according to the results of bioinformatic research. Metformin has ability to inhibit the proliferation of A549 cells, according to the results of colony formation and proliferation assays. In A549 cells, metformin increased glucose uptake and lactate generation, while decreasing ATP synthesis and the NAD + /NADH ratio. In summary, PTEN and mTOR may be potential targets of metformin for the treatment of COVID-19/ LUAD. The mechanism by which metformin inhibits lung adenocarcinoma cell proliferation may be related to glucose metabolism regulated by PI3K/AKT signalling and mTOR signalling pathways. Our study provides a new theoretical basis for the treatment of COVID-19/LUAD.
Ethics approval and consent to participate This study did not require ethical board approval because it did not include human or animal trials.
Author contributions Bin Zhang: Conceptualization. Baoli Xiang: Methodology, Software. Yongwang Hou: Data curation, Writing-Original draft preparation, funding acquisition. Jiangmin Liu: Visualization, Investigation. Lina Geng and Yuhuan Xu: Supervision. Dandan Xu and Minghua Zhan: Software, Validation. Zhicong Yang: Writing-Reviewing and Editing. All authors have reviewed the results and approved the final version of the manuscript.
Competing interests The authors declare no competing interests.
Additional information
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1038/ s41598-024-63081-0. Correspondence and requests for materials should be addressed to Y.H. or B.Z. Reprints and permissions information is available at www.nature.com/reprints. Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ardestani, Azizi, Targeting glucose metabolism for treatment of COVID-19, Signal Transduct. Target. Ther
Bakouny, COVID-19 and cancer: Current challenges and perspectives, Cancer Cell
Benjamin, Dual inhibition of the lactate transporters MCT1 and MCT4 is synthetic lethal with metformin due to NAD+ depletion in cancer cells, Cell Rep
Biray, Sezgin, Goker, Karci, Gode, PI3K/AKT/mTOR pathway and autophagy regulator genes in paranasal squamous cell carcinoma metastasis, Mol. Biol. Rep
Blanco, Cameirao, Lopez, Munoz-Barroso, Phosphatidylinositol-3-kinase-Akt pathway in negative-stranded RNA virus infection: A minireview, Arch. Virol
Boyero, Primary and acquired resistance to immunotherapy in lung cancer: Unveiling the mechanisms underlying of immune checkpoint blockade therapy, Cancers (Basel)
Brancher, Metformin use and lung cancer survival: A population-based study in Norway, Scientific Reports
Che, Intracellular antibody targeting HBx suppresses invasion and metastasis in hepatitis B virus-related hepatocarcinogenesis via protein phosphatase 2A-B56gamma-mediated dephosphorylation of protein kinase B, Cell Prolif
Chen, Chen, He, Stiles, Pten, Tumor suppressor and metabolic regulator, Front. Endocrinol
Cheung, Metformin use and gastric cancer risk in diabetic patients after Helicobacter pylori eradication, J. Natl. Cancer Inst
Deinhardt-Emmer, Inhibition of phosphatidylinositol 3-kinase by pictilisib blocks influenza virus propagation in cells and in lungs of infected mice, Biomolecules
Del, Simvastatin and metformin inhibit cell growth in hepatitis C virus infected cells via mTOR increasing PTEN and autophagy, PLoS One
Dunn, Connor, Dominant inhibition of Akt/protein kinase B signaling by the matrix protein of a negative-strand RNA virus, J. Virol
El-Benhawy, El-Sheredy, Metformin and survival in diabetic patients with breast cancer, J. Egypt Public Health Assoc
Elkrief, Learning through a pandemic: The current state of knowledge on COVID-19 and cancer, Cancer Discov
Ghantous, Hernandez-Vargas, Byrnes, Dwyer, Herceg, Characterising the epigenome as a key component of the fetal exposome in evaluating in utero exposures and childhood cancer risk, Mutagenesis
Goncalves, Hopkins, Cantley, Phosphatidylinositol 3-kinase, growth disorders, and cancer, N. Engl. J. Med
He, Metformin inhibits the migration and invasion of esophageal squamous cell carcinoma cells by downregulating the protein kinase B signaling pathway, Oncol. Lett
Hosp, Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19, Brain
Hu, Flavokawain C inhibits glucose metabolism and tumor angiogenesis in nasopharyngeal carcinoma by targeting the HSP90B1/STAT3/HK2 signaling axis, Cancer Cell Int
Hu, Three subtypes of lung cancer fibroblasts define distinct therapeutic paradigms, Cancer Cell
Hua, Targeting mTOR for cancer therapy, J. Hematol. Oncol
Jin, Metformin-repressed miR-381-YAP-snail axis activity disrupts NSCLC growth and metastasis, J. Exp. Clin. Cancer Res
Kang, The associations of aspirin, statins, and metformin with lung cancer risk and related mortality: A time-dependent analysis of population-based nationally representative data, J. Thorac. Oncol
Kim, Metformin use reduced the overall risk of cancer in diabetic patients: A study based on the Korean NHIS-HEALS cohort, Nutr. Metab. Cardiovasc. Dis
Li, Metformin in patients with COVID-19: A systematic review and meta-analysis, Front. Med
Liang, Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China, Lancet Oncol
Liu, Cao, Glucose metabolism of TAMs in tumor chemoresistance and metastasis, Trends Cell Biol
Lu, Metformin inhibits human non-small cell lung cancer by regulating AMPK-CEBPB-PDL1 signaling pathway, Cancer Immunol. Immunother
Ma, Patel, Vemparala, Krishnamurthy, Metformin is associated with favorable outcomes in patients with COVID-19 and type 2 diabetes mellitus, Sci. Rep
Ma, Zheng, Xiao, Zhou, Tan, Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients, Medicine (Baltimore)
Majumder, Minko, Recent developments on therapeutic and diagnostic approaches for COVID-19, AAPS J
Moreira, The redox status of cancer cells supports mechanisms behind the Warburg effect, Metabolites
Moubarak, COVID-19 and lung cancer: Update on the latest screening, diagnosis, management and challenges, J. Int. Med. Res
Musika, Kamsa-Ard, Jirapornkul, Santong, Phunmanee, Lung cancer survival with current therapies and new targeted treatments: A comprehensive update from the Srinagarind Hospital-based cancer registry from (2013 to 2017), Asian Pac. J. Cancer Prev
Navas, Carnero, NAD(+) metabolism, stemness, the immune response, and cancer, Signal Transduct. Target. Ther
Ndembe, Caloric restriction and metformin selectively improved LKB1-mutated NSCLC tumor response to chemo-and chemo-immunotherapy, J. Exp. Clin. Cancer Res
Qian, Metformin suppresses tumor angiogenesis and enhances the chemosensitivity of gemcitabine in a genetically engineered mouse model of pancreatic cancer, Life Sci
Roncon, Zuin, Rigatelli, Zuliani, Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome, J. Clin. Virol
Saygili, Karakilic, Mert, Sener, Mirci, Preadmission usage of metformin and mortality in COVID-19 patients including the post-discharge period, Ir. J. Med. Sci
Scheen, Metformin and COVID-19: From cellular mechanisms to reduced mortality, Diabetes Metab
Scott, Hann, Immunotherapy for small cell lung cancer: Established applications and novel approaches, Clin. Adv. Hematol. Oncol
She, Cost-effectiveness analysis of pembrolizumab versus chemotherapy as first-line treatment in locally advanced or metastatic non-small cell lung cancer with PD-L1 tumor proportion score 1% or greater, Lung Cancer
Strang, Schultz, Dying with cancer and COVID-19, with special reference to lung cancer: Frailty as a risk factor, Cancers (Basel)
Usman, Metformin use in patients hospitalized with COVID-19: Lower inflammation, oxidative stress, and thrombotic risk markers and better clinical outcomes, J. Thromb. Thrombolysis
Wang, Zhang, Du, Li, Li, Fuzzy planar cell polarity gene (FUZ) promtes cell glycolysis, migration, and invasion in non-small cell lung cancer via the phosphoinositide 3-kinase/protein kinase B pathway, J. Cancer
Zheng, Schultz, Sinclair, NAD(+) in COVID-19 and viral infections, Trends Immunol
Zhou, Zhou, Immunotherapy in non-small cell lung cancer: Advancements and challenges, Chin. Med. J. (Engl.)
DOI record:
{
"DOI": "10.1038/s41598-024-63081-0",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-024-63081-0",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Lung adenocarcinoma (LUAD) is the most common and aggressive subtype of lung cancer, and coronavirus disease 2019 (COVID-19) has become a serious public health threat worldwide. Patients with LUAD and COVID-19 have a poor prognosis. Therefore, finding medications that can be used to treat COVID-19/LUAD patients is essential. Bioinformatics analysis was used to identify 20 possible metformin target genes for the treatment of COVID-19/LUAD. PTEN and mTOR may serve as hub target genes of metformin. Metformin may be able to cure COVID-19/LUAD comorbidity through energy metabolism, oxidoreductase NADH activity, FoxO signalling pathway, AMPK signalling system, and mTOR signalling pathway, among other pathways, according to the results of bioinformatic research. Metformin has ability to inhibit the proliferation of A549 cells, according to the results of colony formation and proliferation assays. In A549 cells, metformin increased glucose uptake and lactate generation, while decreasing ATP synthesis and the NAD<jats:sup>+</jats:sup>/NADH ratio. In summary, PTEN and mTOR may be potential targets of metformin for the treatment of COVID-19/LUAD. The mechanism by which metformin inhibits lung adenocarcinoma cell proliferation may be related to glucose metabolism regulated by PI3K/AKT signalling and mTOR signalling pathways. Our study provides a new theoretical basis for the treatment of COVID-19/LUAD.</jats:p>",
"alternative-id": [
"63081"
],
"article-number": "12406",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "13 March 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "24 May 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "30 May 2024"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Hou",
"given": "Yongwang",
"sequence": "first"
},
{
"affiliation": [],
"family": "Yang",
"given": "Zhicong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xiang",
"given": "Baoli",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Jiangmin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Geng",
"given": "Lina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xu",
"given": "Dandan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhan",
"given": "Minghua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xu",
"given": "Yuhuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Bin",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
5,
30
]
],
"date-time": "2024-05-30T03:51:11Z",
"timestamp": 1717041071000
},
"deposited": {
"date-parts": [
[
2024,
5,
30
]
],
"date-time": "2024-05-30T04:15:19Z",
"timestamp": 1717042519000
},
"funder": [
{
"award": [
"20220596"
],
"name": "Hebei Health hScientific Research Foundation Project"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
31
]
],
"date-time": "2024-05-31T00:30:47Z",
"timestamp": 1717115447478
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
5,
30
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
5,
30
]
],
"date-time": "2024-05-30T00:00:00Z",
"timestamp": 1717027200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
5,
30
]
],
"date-time": "2024-05-30T00:00:00Z",
"timestamp": 1717027200000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-024-63081-0.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-024-63081-0",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-024-63081-0.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2024,
5,
30
]
]
},
"published-online": {
"date-parts": [
[
2024,
5,
30
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.ccell.2021.09.003",
"author": "H Hu",
"doi-asserted-by": "publisher",
"first-page": "1531",
"journal-title": "Cancer Cell",
"key": "63081_CR1",
"unstructured": "Hu, H. et al. Three subtypes of lung cancer fibroblasts define distinct therapeutic paradigms. Cancer Cell 39, 1531–1547 (2021).",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.31557/APJCP.2021.22.8.2501",
"author": "W Musika",
"doi-asserted-by": "publisher",
"first-page": "2501",
"journal-title": "Asian Pac. J. Cancer Prev.",
"key": "63081_CR2",
"unstructured": "Musika, W., Kamsa-Ard, S., Jirapornkul, C., Santong, C. & Phunmanee, A. Lung cancer survival with current therapies and new targeted treatments: A comprehensive update from the Srinagarind Hospital-based cancer registry from (2013 to 2017). Asian Pac. J. Cancer Prev. 22, 2501–2507 (2021).",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1016/j.lungcan.2019.10.017",
"author": "L She",
"doi-asserted-by": "publisher",
"first-page": "88",
"journal-title": "Lung Cancer",
"key": "63081_CR3",
"unstructured": "She, L. et al. Cost-effectiveness analysis of pembrolizumab versus chemotherapy as first-line treatment in locally advanced or metastatic non-small cell lung cancer with PD-L1 tumor proportion score 1% or greater. Lung Cancer 138, 88–94 (2019).",
"volume": "138",
"year": "2019"
},
{
"author": "SC Scott",
"first-page": "654",
"journal-title": "Clin. Adv. Hematol. Oncol.",
"key": "63081_CR4",
"unstructured": "Scott, S. C. & Hann, C. L. Immunotherapy for small cell lung cancer: Established applications and novel approaches. Clin. Adv. Hematol. Oncol. 19, 654–663 (2021).",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1097/CM9.0000000000001338",
"author": "F Zhou",
"doi-asserted-by": "publisher",
"first-page": "1135",
"journal-title": "Chin. Med. J. (Engl.)",
"key": "63081_CR5",
"unstructured": "Zhou, F. & Zhou, C. C. Immunotherapy in non-small cell lung cancer: Advancements and challenges. Chin. Med. J. (Engl.) 134, 1135–1137 (2021).",
"volume": "134",
"year": "2021"
},
{
"DOI": "10.3390/cancers12123729",
"author": "L Boyero",
"doi-asserted-by": "publisher",
"first-page": "3729",
"journal-title": "Cancers (Basel)",
"key": "63081_CR6",
"unstructured": "Boyero, L. et al. Primary and acquired resistance to immunotherapy in lung cancer: Unveiling the mechanisms underlying of immune checkpoint blockade therapy. Cancers (Basel) 12, 3729 (2020).",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1093/mutage/gev010",
"author": "A Ghantous",
"doi-asserted-by": "publisher",
"first-page": "733",
"journal-title": "Mutagenesis",
"key": "63081_CR7",
"unstructured": "Ghantous, A., Hernandez-Vargas, H., Byrnes, G., Dwyer, T. & Herceg, Z. Characterising the epigenome as a key component of the fetal exposome in evaluating in utero exposures and childhood cancer risk. Mutagenesis 30, 733–742 (2015).",
"volume": "30",
"year": "2015"
},
{
"DOI": "10.1208/s12248-020-00532-2",
"author": "J Majumder",
"doi-asserted-by": "publisher",
"first-page": "14",
"journal-title": "AAPS J.",
"key": "63081_CR8",
"unstructured": "Majumder, J. & Minko, T. Recent developments on therapeutic and diagnostic approaches for COVID-19. AAPS J. 23, 14 (2021).",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1016/S1470-2045(20)30096-6",
"author": "W Liang",
"doi-asserted-by": "publisher",
"first-page": "335",
"journal-title": "Lancet Oncol.",
"key": "63081_CR9",
"unstructured": "Liang, W. et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 21, 335–337 (2020).",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1177/03000605221125047",
"author": "S Moubarak",
"doi-asserted-by": "publisher",
"journal-title": "J. Int. Med. Res.",
"key": "63081_CR10",
"unstructured": "Moubarak, S. et al. COVID-19 and lung cancer: Update on the latest screening, diagnosis, management and challenges. J. Int. Med. Res. 50, 665781321 (2022).",
"volume": "50",
"year": "2022"
},
{
"DOI": "10.3390/cancers14236002",
"author": "P Strang",
"doi-asserted-by": "publisher",
"first-page": "6002",
"journal-title": "Cancers (Basel)",
"key": "63081_CR11",
"unstructured": "Strang, P. & Schultz, T. Dying with cancer and COVID-19, with special reference to lung cancer: Frailty as a risk factor. Cancers (Basel) 14, 6002 (2022).",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1158/2159-8290.CD-21-1368",
"author": "A Elkrief",
"doi-asserted-by": "publisher",
"first-page": "303",
"journal-title": "Cancer Discov.",
"key": "63081_CR12",
"unstructured": "Elkrief, A. et al. Learning through a pandemic: The current state of knowledge on COVID-19 and cancer. Cancer Discov. 12, 303–330 (2022).",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1016/j.jcv.2020.104354",
"author": "L Roncon",
"doi-asserted-by": "publisher",
"journal-title": "J. Clin. Virol.",
"key": "63081_CR13",
"unstructured": "Roncon, L., Zuin, M., Rigatelli, G. & Zuliani, G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J. Clin. Virol. 127, 104354 (2020).",
"volume": "127",
"year": "2020"
},
{
"DOI": "10.1016/j.numecd.2020.05.010",
"author": "YS Kim",
"doi-asserted-by": "publisher",
"first-page": "1714",
"journal-title": "Nutr. Metab. Cardiovasc. Dis.",
"key": "63081_CR14",
"unstructured": "Kim, Y. S. et al. Metformin use reduced the overall risk of cancer in diabetic patients: A study based on the Korean NHIS-HEALS cohort. Nutr. Metab. Cardiovasc. Dis. 30, 1714–1722 (2020).",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1093/jnci/djy144",
"author": "KS Cheung",
"doi-asserted-by": "publisher",
"first-page": "484",
"journal-title": "J. Natl. Cancer Inst.",
"key": "63081_CR15",
"unstructured": "Cheung, K. S. et al. Metformin use and gastric cancer risk in diabetic patients after Helicobacter pylori eradication. J. Natl. Cancer Inst. 111, 484–489 (2019).",
"volume": "111",
"year": "2019"
},
{
"DOI": "10.1097/MD.0000000000006888",
"author": "S Ma",
"doi-asserted-by": "publisher",
"journal-title": "Medicine (Baltimore)",
"key": "63081_CR16",
"unstructured": "Ma, S., Zheng, Y., Xiao, Y., Zhou, P. & Tan, H. Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients. Medicine (Baltimore) 96, e6888 (2017).",
"volume": "96",
"year": "2017"
},
{
"DOI": "10.1097/01.EPX.0000456620.00173.c0",
"author": "SA El-Benhawy",
"doi-asserted-by": "publisher",
"first-page": "148",
"journal-title": "J. Egypt Public Health Assoc.",
"key": "63081_CR17",
"unstructured": "El-Benhawy, S. A. & El-Sheredy, H. G. Metformin and survival in diabetic patients with breast cancer. J. Egypt Public Health Assoc. 89, 148–153 (2014).",
"volume": "89",
"year": "2014"
},
{
"DOI": "10.1016/j.diabet.2020.07.006",
"author": "AJ Scheen",
"doi-asserted-by": "publisher",
"first-page": "423",
"journal-title": "Diabetes Metab.",
"key": "63081_CR18",
"unstructured": "Scheen, A. J. Metformin and COVID-19: From cellular mechanisms to reduced mortality. Diabetes Metab. 46, 423–426 (2020).",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.3389/fmed.2021.704666",
"author": "Y Li",
"doi-asserted-by": "publisher",
"journal-title": "Front. Med. (Lausanne)",
"key": "63081_CR19",
"unstructured": "Li, Y. et al. Metformin in patients with COVID-19: A systematic review and meta-analysis. Front. Med. (Lausanne) 8, 704666 (2021).",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1007/s11845-021-02823-9",
"author": "ES Saygili",
"doi-asserted-by": "publisher",
"first-page": "569",
"journal-title": "Ir. J. Med. Sci.",
"key": "63081_CR20",
"unstructured": "Saygili, E. S., Karakilic, E., Mert, E., Sener, A. & Mirci, A. Preadmission usage of metformin and mortality in COVID-19 patients including the post-discharge period. Ir. J. Med. Sci. 191, 569–575 (2022).",
"volume": "191",
"year": "2022"
},
{
"DOI": "10.1007/s11239-022-02631-7",
"author": "A Usman",
"doi-asserted-by": "publisher",
"first-page": "363",
"journal-title": "J. Thromb. Thrombolysis",
"key": "63081_CR21",
"unstructured": "Usman, A. et al. Metformin use in patients hospitalized with COVID-19: Lower inflammation, oxidative stress, and thrombotic risk markers and better clinical outcomes. J. Thromb. Thrombolysis 53, 363–371 (2022).",
"volume": "53",
"year": "2022"
},
{
"DOI": "10.1007/s00262-021-03116-x",
"author": "T Lu",
"doi-asserted-by": "publisher",
"first-page": "1733",
"journal-title": "Cancer Immunol. Immunother.",
"key": "63081_CR22",
"unstructured": "Lu, T. et al. Metformin inhibits human non-small cell lung cancer by regulating AMPK-CEBPB-PDL1 signaling pathway. Cancer Immunol. Immunother. 71, 1733–1746 (2022).",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1038/s41416-020-01186-9",
"author": "S Brancher",
"doi-asserted-by": "publisher",
"first-page": "1018",
"journal-title": "Br. J. Cancer",
"key": "63081_CR23",
"unstructured": "Brancher, S. et al. Metformin use and lung cancer survival: A population-based study in Norway. Br. J. Cancer 124, 1018–1025 (2021).",
"volume": "124",
"year": "2021"
},
{
"DOI": "10.1016/j.jtho.2020.08.021",
"author": "J Kang",
"doi-asserted-by": "publisher",
"first-page": "76",
"journal-title": "J. Thorac. Oncol.",
"key": "63081_CR24",
"unstructured": "Kang, J. et al. The associations of aspirin, statins, and metformin with lung cancer risk and related mortality: A time-dependent analysis of population-based nationally representative data. J. Thorac. Oncol. 16, 76–88 (2021).",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1038/s41598-022-09639-2",
"author": "Z Ma",
"doi-asserted-by": "publisher",
"journal-title": "Sci. Rep.",
"key": "63081_CR25",
"unstructured": "Ma, Z., Patel, N., Vemparala, P. & Krishnamurthy, M. Metformin is associated with favorable outcomes in patients with COVID-19 and type 2 diabetes mellitus. Sci. Rep. 12, 5553 (2022).",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1016/j.ccell.2020.09.018",
"author": "Z Bakouny",
"doi-asserted-by": "publisher",
"first-page": "629",
"journal-title": "Cancer Cell",
"key": "63081_CR26",
"unstructured": "Bakouny, Z. et al. COVID-19 and cancer: Current challenges and perspectives. Cancer Cell 38, 629–646 (2020).",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.3389/fendo.2018.00338",
"author": "CY Chen",
"doi-asserted-by": "publisher",
"first-page": "338",
"journal-title": "Front. Endocrinol. (Lausanne)",
"key": "63081_CR27",
"unstructured": "Chen, C. Y., Chen, J., He, L. & Stiles, B. L. PTEN: Tumor suppressor and metabolic regulator. Front. Endocrinol. (Lausanne) 9, 338 (2018).",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1186/s13045-019-0754-1",
"author": "H Hua",
"doi-asserted-by": "publisher",
"first-page": "71",
"journal-title": "J. Hematol. Oncol.",
"key": "63081_CR28",
"unstructured": "Hua, H. et al. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 12, 71 (2019).",
"volume": "12",
"year": "2019"
},
{
"author": "CJ Del",
"journal-title": "PLoS One",
"key": "63081_CR29",
"unstructured": "Del, C. J. et al. Simvastatin and metformin inhibit cell growth in hepatitis C virus infected cells via mTOR increasing PTEN and autophagy. PLoS One 13, e191805 (2018).",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1186/s13046-023-02933-5",
"author": "G Ndembe",
"doi-asserted-by": "publisher",
"first-page": "6",
"journal-title": "J. Exp. Clin. Cancer Res.",
"key": "63081_CR30",
"unstructured": "Ndembe, G. et al. Caloric restriction and metformin selectively improved LKB1-mutated NSCLC tumor response to chemo- and chemo-immunotherapy. J. Exp. Clin. Cancer Res. 43, 6 (2024).",
"volume": "43",
"year": "2024"
},
{
"DOI": "10.1186/s13046-019-1503-6",
"author": "D Jin",
"doi-asserted-by": "publisher",
"first-page": "6",
"journal-title": "J. Exp. Clin. Cancer Res.",
"key": "63081_CR31",
"unstructured": "Jin, D. et al. Metformin-repressed miR-381-YAP-snail axis activity disrupts NSCLC growth and metastasis. J. Exp. Clin. Cancer Res. 39, 6 (2020).",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1016/j.lfs.2018.07.046",
"author": "W Qian",
"doi-asserted-by": "publisher",
"first-page": "253",
"journal-title": "Life Sci.",
"key": "63081_CR32",
"unstructured": "Qian, W. et al. Metformin suppresses tumor angiogenesis and enhances the chemosensitivity of gemcitabine in a genetically engineered mouse model of pancreatic cancer. Life Sci. 208, 253–261 (2018).",
"volume": "208",
"year": "2018"
},
{
"DOI": "10.1038/s41392-021-00532-4",
"author": "A Ardestani",
"doi-asserted-by": "publisher",
"first-page": "112",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "63081_CR33",
"unstructured": "Ardestani, A. & Azizi, Z. Targeting glucose metabolism for treatment of COVID-19. Signal Transduct. Target. Ther. 6, 112 (2021).",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1093/brain/awab009",
"author": "JA Hosp",
"doi-asserted-by": "publisher",
"first-page": "1263",
"journal-title": "Brain",
"key": "63081_CR34",
"unstructured": "Hosp, J. A. et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain 144, 1263–1276 (2021).",
"volume": "144",
"year": "2021"
},
{
"DOI": "10.1038/s41392-020-00354-w",
"author": "LE Navas",
"doi-asserted-by": "publisher",
"first-page": "2",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "63081_CR35",
"unstructured": "Navas, L. E. & Carnero, A. NAD(+) metabolism, stemness, the immune response, and cancer. Signal Transduct. Target. Ther. 6, 2 (2021).",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.3390/metabo6040033",
"author": "JD Moreira",
"doi-asserted-by": "publisher",
"first-page": "33",
"journal-title": "Metabolites",
"key": "63081_CR36",
"unstructured": "Moreira, J. D. et al. The redox status of cancer cells supports mechanisms behind the Warburg effect. Metabolites 6, 33 (2016).",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.1007/s11033-020-05458-8",
"author": "AC Biray",
"doi-asserted-by": "publisher",
"first-page": "3641",
"journal-title": "Mol. Biol. Rep.",
"key": "63081_CR37",
"unstructured": "Biray, A. C., Sezgin, B., Goker, B. B., Karci, H. B. & Gode, S. PI3K/AKT/mTOR pathway and autophagy regulator genes in paranasal squamous cell carcinoma metastasis. Mol. Biol. Rep. 47, 3641–3651 (2020).",
"volume": "47",
"year": "2020"
},
{
"DOI": "10.1016/j.tcb.2023.03.008",
"author": "J Liu",
"doi-asserted-by": "publisher",
"first-page": "967",
"journal-title": "Trends Cell Biol.",
"key": "63081_CR38",
"unstructured": "Liu, J. & Cao, X. Glucose metabolism of TAMs in tumor chemoresistance and metastasis. Trends Cell Biol. 33, 967–978 (2023).",
"volume": "33",
"year": "2023"
},
{
"DOI": "10.1186/s12935-024-03314-4",
"author": "Y Hu",
"doi-asserted-by": "publisher",
"first-page": "158",
"journal-title": "Cancer Cell Int.",
"key": "63081_CR39",
"unstructured": "Hu, Y. et al. Flavokawain C inhibits glucose metabolism and tumor angiogenesis in nasopharyngeal carcinoma by targeting the HSP90B1/STAT3/HK2 signaling axis. Cancer Cell Int. 24, 158 (2024).",
"volume": "24",
"year": "2024"
},
{
"DOI": "10.1016/j.it.2022.02.001",
"author": "M Zheng",
"doi-asserted-by": "publisher",
"first-page": "283",
"journal-title": "Trends Immunol.",
"key": "63081_CR40",
"unstructured": "Zheng, M., Schultz, M. B. & Sinclair, D. A. NAD(+) in COVID-19 and viral infections. Trends Immunol. 43, 283–295 (2022).",
"volume": "43",
"year": "2022"
},
{
"DOI": "10.1056/NEJMra1704560",
"author": "MD Goncalves",
"doi-asserted-by": "publisher",
"first-page": "2052",
"journal-title": "N. Engl. J. Med.",
"key": "63081_CR41",
"unstructured": "Goncalves, M. D., Hopkins, B. D. & Cantley, L. C. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N. Engl. J. Med. 379, 2052–2062 (2018).",
"volume": "379",
"year": "2018"
},
{
"DOI": "10.7150/jca.63152",
"author": "S Wang",
"doi-asserted-by": "publisher",
"first-page": "2419",
"journal-title": "J. Cancer",
"key": "63081_CR42",
"unstructured": "Wang, S., Zhang, H., Du, B., Li, X. & Li, Y. Fuzzy planar cell polarity gene (FUZ) promtes cell glycolysis, migration, and invasion in non-small cell lung cancer via the phosphoinositide 3-kinase/protein kinase B pathway. J. Cancer 13, 2419–2429 (2022).",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3390/biom11060808",
"author": "S Deinhardt-Emmer",
"doi-asserted-by": "publisher",
"first-page": "808",
"journal-title": "Biomolecules",
"key": "63081_CR43",
"unstructured": "Deinhardt-Emmer, S. et al. Inhibition of phosphatidylinositol 3-kinase by pictilisib blocks influenza virus propagation in cells and in lungs of infected mice. Biomolecules 11, 808 (2021).",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1007/s00705-020-04740-1",
"author": "J Blanco",
"doi-asserted-by": "publisher",
"first-page": "2165",
"journal-title": "Arch. Virol.",
"key": "63081_CR44",
"unstructured": "Blanco, J., Cameirao, C., Lopez, M. C. & Munoz-Barroso, I. Phosphatidylinositol-3-kinase-Akt pathway in negative-stranded RNA virus infection: A minireview. Arch. Virol. 165, 2165–2176 (2020).",
"volume": "165",
"year": "2020"
},
{
"DOI": "10.1128/JVI.01671-10",
"author": "EF Dunn",
"doi-asserted-by": "publisher",
"first-page": "422",
"journal-title": "J. Virol.",
"key": "63081_CR45",
"unstructured": "Dunn, E. F. & Connor, J. H. Dominant inhibition of Akt/protein kinase B signaling by the matrix protein of a negative-strand RNA virus. J. Virol. 85, 422–431 (2011).",
"volume": "85",
"year": "2011"
},
{
"DOI": "10.1111/cpr.13304",
"author": "L Che",
"doi-asserted-by": "publisher",
"journal-title": "Cell Prolif.",
"key": "63081_CR46",
"unstructured": "Che, L. et al. Intracellular antibody targeting HBx suppresses invasion and metastasis in hepatitis B virus-related hepatocarcinogenesis via protein phosphatase 2A–B56gamma-mediated dephosphorylation of protein kinase B. Cell Prolif. 55, e13304 (2022).",
"volume": "55",
"year": "2022"
},
{
"author": "Y He",
"first-page": "2939",
"journal-title": "Oncol. Lett.",
"key": "63081_CR47",
"unstructured": "He, Y. et al. Metformin inhibits the migration and invasion of esophageal squamous cell carcinoma cells by downregulating the protein kinase B signaling pathway. Oncol. Lett. 15, 2939–2945 (2018).",
"volume": "15",
"year": "2018"
},
{
"DOI": "10.1016/j.celrep.2018.11.043",
"author": "D Benjamin",
"doi-asserted-by": "publisher",
"first-page": "3047",
"journal-title": "Cell Rep.",
"key": "63081_CR48",
"unstructured": "Benjamin, D. et al. Dual inhibition of the lactate transporters MCT1 and MCT4 is synthetic lethal with metformin due to NAD+ depletion in cancer cells. Cell Rep. 25, 3047–3058 (2018).",
"volume": "25",
"year": "2018"
}
],
"reference-count": 48,
"references-count": 48,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-024-63081-0"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Metformin is a potential therapeutic for COVID-19/LUAD by regulating glucose metabolism",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "14"
}