Virologic Outcomes with Molnupiravir in Non-hospitalized Adult Patients with COVID-19 from the Randomized, Placebo-Controlled MOVe-OUT Trial
et al., Infectious Diseases and Therapy, doi:10.1007/s40121-023-00891-1, MOVe-OUT, NCT04575597, Nov 2023
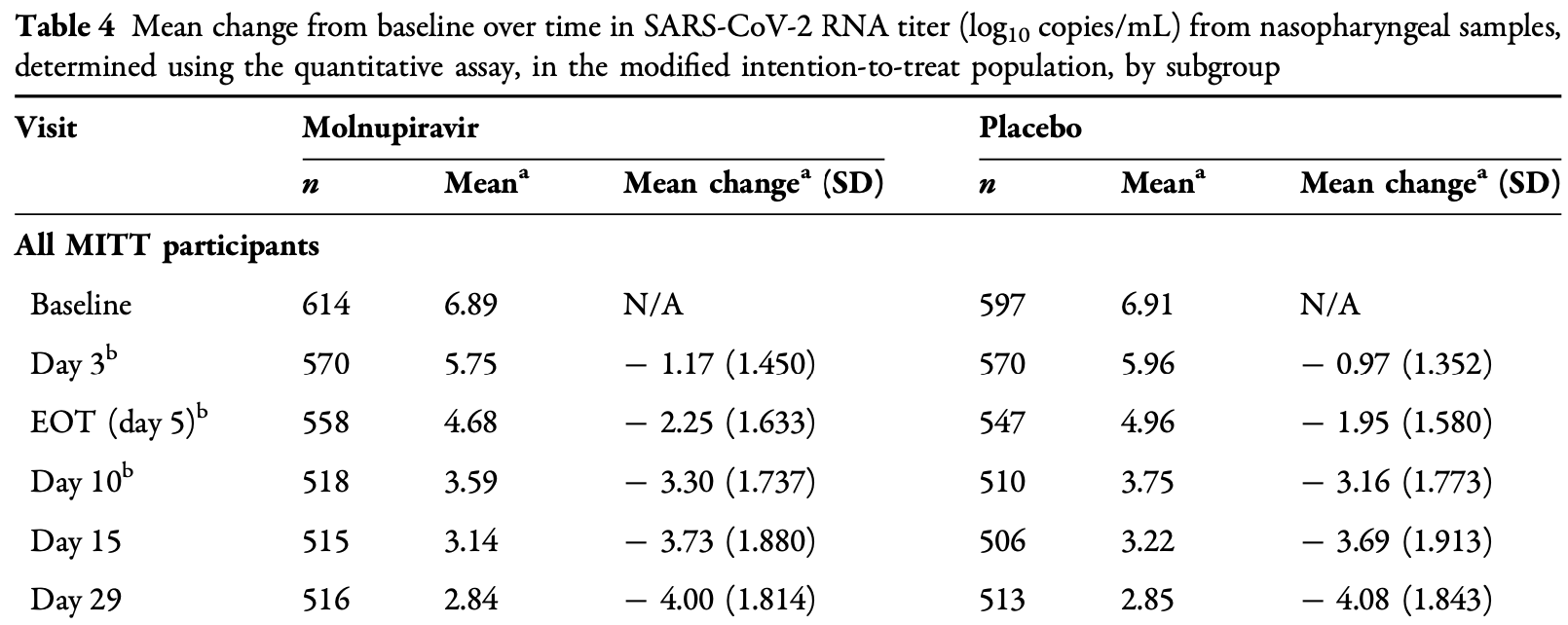
Virological outcomes for the MOVe-OUT trial. Results are shown with the main paper1.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity2-16. Multiple analyses have identified variants potentially created by molnupiravir17-21. Studies show significantly increased risk of acute kidney injury22, cardiovascular toxocity23, and neurological symptoms22. Treatment may increase viral rebound24,25.
1.
Jayk Bernal et al., Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, New England Journal of Medicine, doi:10.1056/NEJMoa2116044.
2.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
3.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
4.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
5.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
6.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
7.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
8.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
9.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
10.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
11.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
12.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
13.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
14.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
15.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
16.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
17.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
18.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
19.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
20.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
22.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
23.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Strizki et al., 23 Nov 2023, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, 10 authors, study period May 2021 - November 2021, average treatment delay 4.0 days, trial NCT04575597 (history) (MOVe-OUT).
Contact: julie.strizki@merck.com.
Virologic Outcomes with Molnupiravir in Non-hospitalized Adult Patients with COVID-19 from the Randomized, Placebo-Controlled MOVe-OUT Trial
Infectious Diseases and Therapy, doi:10.1007/s40121-023-00891-1
Introduction: The randomized, placebo-controlled, double-blind MOVe-OUT trial demonstrated molnupiravir (800 mg every 12 h for 5 days) as safe and effective for outpatient treatment of mild-to-moderate COVID-19, significantly reducing the risk of hospitalization/ death in high-risk adults. At the time of that report, virologic assessments from the trial were partially incomplete as a result of their timeintensive nature. Here we present final results from all prespecified virology endpoints in MOVe-OUT based on the full trial dataset.
Supplementary Information The online version contains supplementary material available at https:// doi.org/10.1007/s40121-023-00891- MSD), at the time the study was conducted. Jiejun Du is currently an employee of Moderna, and Jay A. Grobler is currently an employee of Pfizer Inc.
Ethical Approval. Our company's approach to the conduct of clinical trials is in accordance with the ethical principles that have their origin in the Declaration of Helsinki, and that are consistent with Good Clinical Practice and the applicable regulatory requirement(s). The trial was conducted in accordance with principles of Good Clinical Practice and was approved by the appropriate institutional
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Abdelnabi, Foo, Jonghe, Molnupiravir inhibits the replication of the emerging SARS-CoV-2 variants of concern (VoCs) in a hamster infection model, J Infect Dis
Agostini, Pruijssers, Chappell, Smallmolecule antiviral beta-d-N(4)-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance, J Virol
Aksamentov, Roemer, Hodcroft, Nextclade: clade assignment, mutation calling and quality control for viral genomes, J Open Source Softw
Arribas, Bhagani, Lobo, Randomized trial of molnupiravir or placebo in patients hospitalized with Covid-19, NEJM Evid, doi:10.1056/EVIDoa2100044
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients, N Engl J Med
Boucau, Marino, Regan, Duration of shedding of culturable virus in SARS-CoV-2 Omicron (BA.1) infection, N Engl J Med
Bowe, Xie, Al-Aly, Acute and postacute sequelae associated with SARS-CoV-2 reinfection, Nat Med
Brown, Fu, Bansal, Omicron BA.1/1.1 SARS-CoV-2 infection among vaccinated Canadian adults, N Engl J Med
Butler, Hobbs, Gbinigie, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet
Butt, Talisa, Shaikh, Relative vaccine effectiveness of a SARS-CoV-2 mRNA vaccine booster dose against the Omicron variant, Clin Infect Dis
Caraco, Crofoot, Moncada, Phase 2/3 trial of molnupiravir for treatment of COVID-19 in nonhospitalized adults, NEJM Evid, doi:10.1056/EVIDoa2100043
Cevik, Tate, Llloyd, SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis, Lancet Microbe
Chawla, Birger, Wan, Factors influencing COVID-19 risk: insights from molnupiravir exposure-response modeling of clinical outcomes, Clin Pharmacol Ther
Cox, Wolf, Plemper, Therapeutically administered ribonucleoside analogue MK-4482/ EIDD-2801 blocks SARS-CoV-2 transmission in ferrets, Nat Microbiol
Dal-Re, Becker, Bottieau, Availability of oral antivirals against SARS-CoV-2 infection and the requirement for an ethical prescribing approach, Lancet Infect Dis
Donovan-Banfield, Penrice-Randal, Goldswain, Characterisation of SARS-CoV-2 genomic variation in response to molnupiravir treatment in the AGILE phase IIa clinical trial, Nat Commun
Fischer Wa 2nd, Eron, Jr, Holman, A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus, Sci Transl Med
Goldberg, Mandel, Bar-On, Protection and waning of natural and hybrid immunity to SARS-CoV-2, N Engl J Med
Gordon, Tchesnokov, Schinazi, Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template, J Biol Chem
Grobler, Strizki, Murgolo, Molnupiravir maintains antiviral activity against SARS-CoV-2 variants in vitro and in early clinical studies, Open Forum Infect Dis
Johnson, Puenpatom, Moncada, Effect of molnupiravir on biomarkers, respiratory interventions, and medical services in COVID-19: a randomized, placebo-controlled trial, Ann Intern Med
Jones, Biele, Muhlemann, Estimating infectiousness throughout SARS-CoV-2 infection course, Science, doi:10.1126/science.abi5273
Kabinger, Stiller, Schmitzova, Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis, Nat Struct Mol Biol
Kang, Park, Kim, Comparison of culture-competent virus shedding duration of SARS-CoV-2 Omicron variant in regard to vaccination status: a prospective cohort study, Vaccine
Khoo, Fitzgerald, Saunders, Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): a randomised, placebocontrolled, double-blind, phase 2 trial, Lancet Infect Dis
Levin, Lustig, Cohen, Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months, N Engl J Med
Malone, Campbell, Molnupiravir: coding for catastrophe, Nat Struct Mol Biol
Mazzotta, Lepri, Colavita, Viral load decrease in SARS-CoV-2 BA.1 and BA.2 Omicron sublineages infection after treatment with monoclonal antibodies and direct antiviral agents, J Med Virol
Meng, Abdullahi, Ferreira, Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts tropism and fusogenicity, Nature
Perez-Alos, Armenteros, Madsen, Modeling of waning immunity after SARS-CoV-2 vaccination and influencing factors, Nat Commun
Puhach, Meyer, Eckerle, SARS-CoV-2 viral load and shedding kinetics, Nat Rev Microbiol
Rosenke, Okumura, Lewis, Molnupiravir inhibits SARS-CoV-2 variants including Omicron in the hamster model, JCI Insight
Sethuraman, Jeremiah, Ryo, Interpreting diagnostic tests for SARS-CoV-2, JAMA
Sheahan, Sims, Zhou, An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice, Sci Transl Med
Singh, Stellrecht, Arunachalam, Lack of active SARS-CoV-2 virus in a subset of PCR-positive COVID-19 congregate care patients, J Clin Virol
Sonnleitner, Dorighi, Jansen, An in vitro model for assessment of SARS-CoV-2 infectivity by defining the correlation between virus isolation and quantitative PCR value: isolation success of SARS-CoV-2 from oropharyngeal swabs correlates negatively with Cq value, Virol J
Ssentongo, Ssentongo, Voleti, SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: a systematic review and meta-analysis, BMC Infect Dis
Takashita, Kinoshita, Yamayoshi, Efficacy of antibodies and antiviral drugs against Covid-19 Omicron variant, N Engl J Med
Takashita, Yamayoshi, Simon, Efficacy of antibodies and antiviral drugs against Omicron BA. 2.12.1, BA.4, and BA.5 subvariants, N Engl J Med
Uraki, Kiso, Iida, Characterization and antiviral susceptibility of SARS-CoV-2 Omicron/BA. 2, Nature
Urakova, Kuznetsova, Crossman, b-d-N4-Hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome, J Virol
Vangeel, Chiu, Jonghe, Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern, Antiviral Res
Wahl, Gralinski, Johnson, SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801, Nature
Wai, Chan, Cheung, Association of molnupiravir and nirmatrelvir-ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19, Lancet Reg Health West Pac
Wo ¨lfel, Corman, Guggemos, Virological assessment of hospitalized patients with COVID-2019, Nature
Wong, Au, Lau, Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and inhospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the Omicron wave in Hong Kong: an observational study, Lancet
Xie, Bowe, Al-Aly, Molnupiravir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records, BMJ
Xie, Choi, Al-Aly, Molnupiravir and risk of post-acute sequelae of Covid-19: cohort study, BMJ
Yoon, Toots, Lee, Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses, Antimicrob Agents Chemother
DOI record:
{
"DOI": "10.1007/s40121-023-00891-1",
"ISSN": [
"2193-8229",
"2193-6382"
],
"URL": "http://dx.doi.org/10.1007/s40121-023-00891-1",
"alternative-id": [
"891"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "11 August 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "31 October 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "23 November 2023"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of Interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Julie M. Strizki, Nicholas Murgolo, Arthur Fridman, Matthew G. Johnson, Patricia Carmelitano, Michelle L. Brown, Amanda Paschke, and Carisa De Anda are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD), who may own stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. Jiejun Du and Jay A. Grobler were employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD), at the time the study was conducted. Jiejun Du is currently an employee of Moderna, and Jay A. Grobler is currently an employee of Pfizer Inc."
},
{
"group": {
"label": "Ethical Approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Our company’s approach to the conduct of clinical trials is in accordance with the ethical principles that have their origin in the Declaration of Helsinki, and that are consistent with Good Clinical Practice and the applicable regulatory requirement(s). The trial was conducted in accordance with principles of Good Clinical Practice and was approved by the appropriate institutional review boards/ethics committees and regulatory agencies at all institutions/study sites (see Table S8). Written informed consent was provided by all participants prior to their enrollment into the trial. No identifying information for any trial participant is included in the manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship on this publication, take responsibility for the integrity of the work as a whole, and have given their approval for this final version to be published."
}
],
"author": [
{
"ORCID": "http://orcid.org/0009-0002-3893-9374",
"affiliation": [],
"authenticated-orcid": false,
"family": "Strizki",
"given": "Julie M.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Grobler",
"given": "Jay A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murgolo",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fridman",
"given": "Arthur",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Johnson",
"given": "Matthew G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Du",
"given": "Jiejun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carmelitano",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brown",
"given": "Michelle L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paschke",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Anda",
"given": "Carisa",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04575597",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "Infectious Diseases and Therapy",
"container-title-short": "Infect Dis Ther",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2023,
11,
23
]
],
"date-time": "2023-11-23T06:01:41Z",
"timestamp": 1700719301000
},
"deposited": {
"date-parts": [
[
2023,
11,
23
]
],
"date-time": "2023-11-23T07:12:43Z",
"timestamp": 1700723563000
},
"funder": [
{
"name": "Merck & Co, Inc."
}
],
"indexed": {
"date-parts": [
[
2023,
11,
24
]
],
"date-time": "2023-11-24T00:19:33Z",
"timestamp": 1700785173701
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
11,
23
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
23
]
],
"date-time": "2023-11-23T00:00:00Z",
"timestamp": 1700697600000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
23
]
],
"date-time": "2023-11-23T00:00:00Z",
"timestamp": 1700697600000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-023-00891-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s40121-023-00891-1/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-023-00891-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2023,
11,
23
]
]
},
"published-online": {
"date-parts": [
[
2023,
11,
23
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "891_CR1",
"unstructured": "World Health Organization. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/. Accessed 24 Sept 2023."
},
{
"DOI": "10.1038/s41467-022-29225-4",
"author": "L Perez-Alos",
"doi-asserted-by": "publisher",
"first-page": "1614",
"issue": "1",
"journal-title": "Nat Commun",
"key": "891_CR2",
"unstructured": "Perez-Alos L, Armenteros JJA, Madsen JR, et al. Modeling of waning immunity after SARS-CoV-2 vaccination and influencing factors. Nat Commun. 2022;13(1):1614.",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2114583",
"author": "EG Levin",
"doi-asserted-by": "publisher",
"issue": "24",
"journal-title": "N Engl J Med",
"key": "891_CR3",
"unstructured": "Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84.",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1186/s12879-022-07418-y",
"author": "P Ssentongo",
"doi-asserted-by": "publisher",
"first-page": "439",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "891_CR4",
"unstructured": "Ssentongo P, Ssentongo AE, Voleti N, et al. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2022;22(1):439.",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac328",
"author": "AA Butt",
"doi-asserted-by": "publisher",
"first-page": "2161",
"issue": "12",
"journal-title": "Clin Infect Dis",
"key": "891_CR5",
"unstructured": "Butt AA, Talisa VB, Shaikh OS, et al. Relative vaccine effectiveness of a SARS-CoV-2 mRNA vaccine booster dose against the Omicron variant. Clin Infect Dis. 2022;75(12):2161–8.",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2202879",
"author": "PE Brown",
"doi-asserted-by": "publisher",
"first-page": "2337",
"issue": "24",
"journal-title": "N Engl J Med",
"key": "891_CR6",
"unstructured": "Brown PE, Fu SH, Bansal A, et al. Omicron BA.1/1.1 SARS-CoV-2 infection among vaccinated Canadian adults. N Engl J Med. 2022;386(24):2337–9.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118946",
"author": "Y Goldberg",
"doi-asserted-by": "publisher",
"first-page": "2201",
"issue": "23",
"journal-title": "N Engl J Med",
"key": "891_CR7",
"unstructured": "Goldberg Y, Mandel M, Bar-On YM, et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N Engl J Med. 2022;386(23):2201–12.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41591-022-02051-3",
"author": "B Bowe",
"doi-asserted-by": "publisher",
"first-page": "2398",
"issue": "11",
"journal-title": "Nat Med",
"key": "891_CR8",
"unstructured": "Bowe B, Xie Y, Al-Aly Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med. 2022;28(11):2398–405.",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"author": "CC Butler",
"doi-asserted-by": "publisher",
"first-page": "281",
"issue": "10373",
"journal-title": "Lancet",
"key": "891_CR9",
"unstructured": "Butler CC, Hobbs FDR, Gbinigie OA, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet. 2023;401(10373):281–93.",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(22)00119-0",
"author": "R Dal-Re",
"doi-asserted-by": "publisher",
"first-page": "e231",
"issue": "8",
"journal-title": "Lancet Infect Dis",
"key": "891_CR10",
"unstructured": "Dal-Re R, Becker SL, Bottieau E, et al. Availability of oral antivirals against SARS-CoV-2 infection and the requirement for an ethical prescribing approach. Lancet Infect Dis. 2022;22(8):e231–8.",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1128/AAC.00766-18",
"author": "JJ Yoon",
"doi-asserted-by": "publisher",
"first-page": "e00766",
"issue": "8",
"journal-title": "Antimicrob Agents Chemother",
"key": "891_CR11",
"unstructured": "Yoon JJ, Toots M, Lee S, et al. Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses. Antimicrob Agents Chemother. 2018;62(8):e00766-78.",
"volume": "62",
"year": "2018"
},
{
"DOI": "10.1038/s41564-020-00835-2",
"author": "RM Cox",
"doi-asserted-by": "publisher",
"first-page": "11",
"issue": "1",
"journal-title": "Nat Microbiol",
"key": "891_CR12",
"unstructured": "Cox RM, Wolf JD, Plemper RK. Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat Microbiol. 2021;6(1):11–8.",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1126/scitranslmed.abb5883",
"author": "TP Sheahan",
"doi-asserted-by": "publisher",
"first-page": "eabb5883",
"issue": "541",
"journal-title": "Sci Transl Med",
"key": "891_CR13",
"unstructured": "Sheahan TP, Sims AC, Zhou S, et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020;12(541):eabb5883.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1038/s41586-021-03312-w",
"author": "A Wahl",
"doi-asserted-by": "publisher",
"first-page": "451",
"journal-title": "Nature",
"key": "891_CR14",
"unstructured": "Wahl A, Gralinski LE, Johnson CE, et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;591:451–7.",
"volume": "591",
"year": "2021"
},
{
"DOI": "10.1093/infdis/jiab361",
"author": "R Abdelnabi",
"doi-asserted-by": "publisher",
"first-page": "749",
"issue": "5",
"journal-title": "J Infect Dis",
"key": "891_CR15",
"unstructured": "Abdelnabi R, Foo CS, De Jonghe S, et al. Molnupiravir inhibits the replication of the emerging SARS-CoV-2 variants of concern (VoCs) in a hamster infection model. J Infect Dis. 2021;224(5):749–53.",
"volume": "224",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2022.105252",
"author": "L Vangeel",
"doi-asserted-by": "publisher",
"journal-title": "Antiviral Res",
"key": "891_CR16",
"unstructured": "Vangeel L, Chiu W, De Jonghe S, et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antiviral Res. 2022;198: 105252.",
"volume": "198",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04474-x",
"author": "B Meng",
"doi-asserted-by": "publisher",
"first-page": "706",
"issue": "7902",
"journal-title": "Nature",
"key": "891_CR17",
"unstructured": "Meng B, Abdullahi A, Ferreira I, et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts tropism and fusogenicity. Nature. 2022;603(7902):706–14.",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2119407",
"author": "E Takashita",
"doi-asserted-by": "publisher",
"first-page": "995",
"issue": "10",
"journal-title": "N Engl J Med",
"key": "891_CR18",
"unstructured": "Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antibodies and antiviral drugs against Covid-19 Omicron variant. N Engl J Med. 2022;386(10):995–8.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04856-1",
"author": "R Uraki",
"doi-asserted-by": "publisher",
"first-page": "119",
"issue": "7917",
"journal-title": "Nature",
"key": "891_CR19",
"unstructured": "Uraki R, Kiso M, Iida S, et al. Characterization and antiviral susceptibility of SARS-CoV-2 Omicron/BA.2. Nature. 2022;607(7917):119–27.",
"volume": "607",
"year": "2022"
},
{
"DOI": "10.1172/jci.insight.160108",
"author": "K Rosenke",
"doi-asserted-by": "publisher",
"issue": "13",
"journal-title": "JCI Insight",
"key": "891_CR20",
"unstructured": "Rosenke K, Okumura A, Lewis MC, et al. Molnupiravir inhibits SARS-CoV-2 variants including Omicron in the hamster model. JCI Insight. 2022;7(13):e160108.",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1128/JVI.01348-19",
"author": "ML Agostini",
"doi-asserted-by": "publisher",
"first-page": "e01348",
"issue": "24",
"journal-title": "J Virol",
"key": "891_CR21",
"unstructured": "Agostini ML, Pruijssers AJ, Chappell JD, et al. Small-molecule antiviral beta-d-N(4)-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J Virol. 2019;93(24):e01348–19.",
"volume": "93",
"year": "2019"
},
{
"DOI": "10.1128/JVI.01965-17",
"author": "N Urakova",
"doi-asserted-by": "publisher",
"first-page": "e01965",
"issue": "3",
"journal-title": "J Virol",
"key": "891_CR22",
"unstructured": "Urakova N, Kuznetsova V, Crossman DK, et al. β-d-N4-Hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome. J Virol. 2018;92(3):e01965–17.",
"volume": "92",
"year": "2018"
},
{
"DOI": "10.1093/ofid/ofab466.742",
"author": "J Grobler",
"doi-asserted-by": "publisher",
"first-page": "S373",
"issue": "Suppl 1",
"journal-title": "Open Forum Infect Dis",
"key": "891_CR23",
"unstructured": "Grobler J, Strizki J, Murgolo N, et al. Molnupiravir maintains antiviral activity against SARS-CoV-2 variants in vitro and in early clinical studies. Open Forum Infect Dis. 2021;8(Suppl 1):S373.",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1038/s41594-021-00651-0",
"author": "F Kabinger",
"doi-asserted-by": "publisher",
"first-page": "740",
"journal-title": "Nat Struct Mol Biol",
"key": "891_CR24",
"unstructured": "Kabinger F, Stiller C, Schmitzova J, et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol. 2021;28:740–6.",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1016/j.jbc.2021.100770",
"author": "CJ Gordon",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "J Biol Chem",
"key": "891_CR25",
"unstructured": "Gordon CJ, Tchesnokov EP, Schinazi RF, et al. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J Biol Chem. 2021;297(1):100770.",
"volume": "297",
"year": "2021"
},
{
"DOI": "10.1038/s41594-021-00657-8",
"author": "B Malone",
"doi-asserted-by": "publisher",
"first-page": "706",
"issue": "9",
"journal-title": "Nat Struct Mol Biol",
"key": "891_CR26",
"unstructured": "Malone B, Campbell EA. Molnupiravir: coding for catastrophe. Nat Struct Mol Biol. 2021;28(9):706–8.",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116044",
"author": "A Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "891_CR27",
"unstructured": "Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–20.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.7326/M22-0729",
"author": "MG Johnson",
"doi-asserted-by": "publisher",
"first-page": "1126",
"issue": "8",
"journal-title": "Ann Intern Med",
"key": "891_CR28",
"unstructured": "Johnson MG, Puenpatom A, Moncada PA, et al. Effect of molnupiravir on biomarkers, respiratory interventions, and medical services in COVID-19: a randomized, placebo-controlled trial. Ann Intern Med. 2022;175(8):1126–34.",
"volume": "175",
"year": "2022"
},
{
"DOI": "10.1056/EVIDoa2100043",
"author": "Y Caraco",
"doi-asserted-by": "publisher",
"journal-title": "NEJM Evid",
"key": "891_CR29",
"unstructured": "Caraco Y, Crofoot GE, Moncada PA, et al. Phase 2/3 trial of molnupiravir for treatment of COVID-19 in nonhospitalized adults. NEJM Evid. 2022. https://doi.org/10.1056/EVIDoa2100043.",
"year": "2022"
},
{
"DOI": "10.1186/s12985-021-01542-y",
"author": "ST Sonnleitner",
"doi-asserted-by": "publisher",
"first-page": "71",
"issue": "1",
"journal-title": "Virol J",
"key": "891_CR30",
"unstructured": "Sonnleitner ST, Dorighi J, Jansen B, et al. An in vitro model for assessment of SARS-CoV-2 infectivity by defining the correlation between virus isolation and quantitative PCR value: isolation success of SARS-CoV-2 from oropharyngeal swabs correlates negatively with Cq value. Virol J. 2021;18(1):71.",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1016/j.jcv.2021.104879",
"author": "AK Singh",
"doi-asserted-by": "publisher",
"journal-title": "J Clin Virol",
"key": "891_CR31",
"unstructured": "Singh AK, Stellrecht KA, Arunachalam T, et al. Lack of active SARS-CoV-2 virus in a subset of PCR-positive COVID-19 congregate care patients. J Clin Virol. 2021;141:104879.",
"volume": "141",
"year": "2021"
},
{
"DOI": "10.1126/science.abi5273",
"author": "TC Jones",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "891_CR32",
"unstructured": "Jones TC, Biele G, Muhlemann B, et al. Estimating infectiousness throughout SARS-CoV-2 infection course. Science. 2021. https://doi.org/10.1126/science.abi5273.",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"author": "R Wölfel",
"doi-asserted-by": "publisher",
"first-page": "465",
"issue": "7809",
"journal-title": "Nature",
"key": "891_CR33",
"unstructured": "Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–9.",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.21105/joss.03773",
"author": "I Aksamentov",
"doi-asserted-by": "publisher",
"first-page": "3773",
"issue": "67",
"journal-title": "J Open Source Softw",
"key": "891_CR34",
"unstructured": "Aksamentov I, Roemer C, Hodcroft EB, et al. Nextclade: clade assignment, mutation calling and quality control for viral genomes. J Open Source Softw. 2021;6(67):3773.",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(22)00644-2",
"author": "SH Khoo",
"doi-asserted-by": "publisher",
"first-page": "183",
"issue": "2",
"journal-title": "Lancet Infect Dis",
"key": "891_CR35",
"unstructured": "Khoo SH, FitzGerald R, Saunders G, et al. Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Infect Dis. 2023;23(2):183–95.",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1038/s41467-022-34839-9",
"author": "I Donovan-Banfield",
"doi-asserted-by": "publisher",
"first-page": "7284",
"issue": "1",
"journal-title": "Nat Commun",
"key": "891_CR36",
"unstructured": "Donovan-Banfield I, Penrice-Randal R, Goldswain H, et al. Characterisation of SARS-CoV-2 genomic variation in response to molnupiravir treatment in the AGILE phase IIa clinical trial. Nat Commun. 2022;13(1):7284.",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"author": "WA Fischer 2nd",
"doi-asserted-by": "publisher",
"first-page": "eab17430",
"issue": "628",
"journal-title": "Sci Transl Med",
"key": "891_CR37",
"unstructured": "Fischer WA 2nd, Eron JJ Jr, Holman W, et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci Transl Med. 2022;14(628):eab17430.",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1002/cpt.2895",
"author": "A Chawla",
"doi-asserted-by": "publisher",
"first-page": "1337",
"issue": "6",
"journal-title": "Clin Pharmacol Ther",
"key": "891_CR38",
"unstructured": "Chawla A, Birger R, Wan H, et al. Factors influencing COVID-19 risk: insights from molnupiravir exposure-response modeling of clinical outcomes. Clin Pharmacol Ther. 2023;113(6):1337–45.",
"volume": "113",
"year": "2023"
},
{
"DOI": "10.1001/jama.2020.8259",
"author": "N Sethuraman",
"doi-asserted-by": "publisher",
"first-page": "2249",
"issue": "22",
"journal-title": "JAMA",
"key": "891_CR39",
"unstructured": "Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323(22):2249–51.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/S2666-5247(20)30172-5",
"author": "M Cevik",
"doi-asserted-by": "publisher",
"first-page": "e13",
"issue": "1",
"journal-title": "Lancet Microbe",
"key": "891_CR40",
"unstructured": "Cevik M, Tate M, Llloyd O, et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2(1):e13–22.",
"volume": "2",
"year": "2021"
},
{
"author": "O Puhach",
"first-page": "147",
"issue": "3",
"journal-title": "Nat Rev Microbiol",
"key": "891_CR41",
"unstructured": "Puhach O, Meyer B, Eckerle I. SARS-CoV-2 viral load and shedding kinetics. Nat Rev Microbiol. 2023;21(3):147–61.",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1016/j.vaccine.2023.03.044",
"author": "S-W Kang",
"doi-asserted-by": "publisher",
"first-page": "2769",
"issue": "17",
"journal-title": "Vaccine",
"key": "891_CR42",
"unstructured": "Kang S-W, Park H, Kim JY, et al. Comparison of culture-competent virus shedding duration of SARS-CoV-2 Omicron variant in regard to vaccination status: a prospective cohort study. Vaccine. 2023;41(17):2769–72.",
"volume": "41",
"year": "2023"
},
{
"DOI": "10.1056/NEJMc2202092",
"author": "J Boucau",
"doi-asserted-by": "publisher",
"first-page": "275",
"issue": "3",
"journal-title": "N Engl J Med",
"key": "891_CR43",
"unstructured": "Boucau J, Marino C, Regan J, et al. Duration of shedding of culturable virus in SARS-CoV-2 Omicron (BA.1) infection. N Engl J Med. 2022;387(3):275–7.",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2207519",
"author": "E Takashita",
"doi-asserted-by": "publisher",
"first-page": "468",
"issue": "5",
"journal-title": "N Engl J Med",
"key": "891_CR44",
"unstructured": "Takashita E, Yamayoshi S, Simon V, et al. Efficacy of antibodies and antiviral drugs against Omicron BA.2.12.1, BA.4, and BA.5 subvariants. N Engl J Med. 2022;387(5):468–70.",
"volume": "387",
"year": "2022"
},
{
"key": "891_CR45",
"unstructured": "European Medicines Agency. Withdrawal assessment report for Lagevrio. First published 08 September, 2023. Amsterdam: EMA. 2023. https://www.ema.europa.eu/en/medicines/human/withdrawn-applications/lagevrio. Accessed 24 Sept 2023."
},
{
"DOI": "10.1056/EVIDoa2100044",
"author": "JR Arribas",
"doi-asserted-by": "publisher",
"journal-title": "NEJM Evid",
"key": "891_CR46",
"unstructured": "Arribas JR, Bhagani S, Lobo SM, et al. Randomized trial of molnupiravir or placebo in patients hospitalized with Covid-19. NEJM Evid. 2022. https://doi.org/10.1056/EVIDoa2100044.",
"year": "2022"
},
{
"DOI": "10.1136/bmj-2022-072705",
"author": "Y Xie",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "891_CR47",
"unstructured": "Xie Y, Bowe B, Al-Aly Z. Molnupiravir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ. 2023;380: e072705.",
"volume": "380",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(22)01586-0",
"author": "CHK Wong",
"doi-asserted-by": "publisher",
"first-page": "1213",
"issue": "10359",
"journal-title": "Lancet",
"key": "891_CR48",
"unstructured": "Wong CHK, Au ICH, Lau KTK, et al. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the Omicron wave in Hong Kong: an observational study. Lancet. 2022;400(10359):1213–22.",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1016/j.lanwpc.2022.100602",
"author": "AK Wai",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Reg Health West Pac",
"key": "891_CR49",
"unstructured": "Wai AK, Chan CY, Cheung AW, et al. Association of molnupiravir and nirmatrelvir-ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. Lancet Reg Health West Pac. 2023;30: 100602.",
"volume": "30",
"year": "2023"
},
{
"DOI": "10.1136/bmj-2022-074572",
"author": "Y Xie",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "891_CR50",
"unstructured": "Xie Y, Choi T, Al-Aly Z. Molnupiravir and risk of post-acute sequelae of Covid-19: cohort study. BMJ. 2023;381:e074572.",
"volume": "381",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28186",
"author": "V Mazzotta",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "J Med Virol",
"key": "891_CR51",
"unstructured": "Mazzotta V, Lepri AC, Colavita F, et al. Viral load decrease in SARS-CoV-2 BA.1 and BA.2 Omicron sublineages infection after treatment with monoclonal antibodies and direct antiviral agents. J Med Virol. 2023;95(1):e28186.",
"volume": "95",
"year": "2023"
}
],
"reference-count": 51,
"references-count": 51,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s40121-023-00891-1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Virologic Outcomes with Molnupiravir in Non-hospitalized Adult Patients with COVID-19 from the Randomized, Placebo-Controlled MOVe-OUT Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}