Longitudinal outcomes of COVID-19 in solid organ transplant recipients from 2020 to 2023
et al., American Journal of Transplantation, doi:10.1016/j.ajt.2024.03.011, Mar 2024
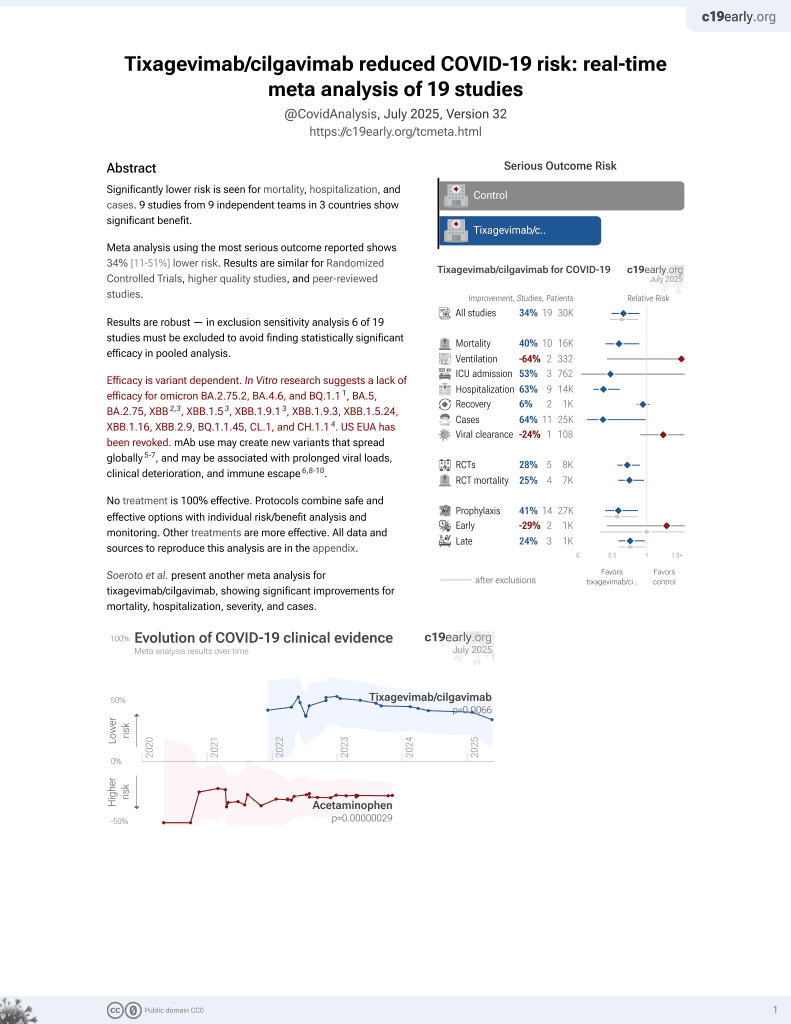
42nd treatment shown to reduce risk in
May 2022, now with p = 0.0066 from 19 studies, recognized in 33 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 1,975 solid organ transplant recipients with COVID-19 showing lower risk of severe cases with tixagevimab/cilgavimab prophylaxis, without statistical significance.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2.75.2, BA.4.6, BQ.1.11, BA.5, BA.2.75, XBB2,3, XBB.1.53, ХВВ.1.9.13, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.14.
|
risk of severe case, 25.6% lower, RR 0.74, p = 0.48, treatment 7 of 156 (4.5%), control 283 of 1,819 (15.6%), NNT 9.0, adjusted per study, odds ratio converted to relative risk, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
2.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Solera et al., 31 Mar 2024, retrospective, Canada, peer-reviewed, median age 57.5, 12 authors.
Contact: jtsolera@gmail.com, deepali.kumar@uhn.ca.
Longitudinal outcomes of COVID-19 in solid organ transplant recipients from 2020 to 2023
American Journal of Transplantation, doi:10.1016/j.ajt.2024.03.011
solid organ transplant outcomes vaccination mRNA vaccines antivirals bivalent booster vaccines remdesivir
Declaration of competing interest Deepali Kumar has received clinical trial grants from GSK, Roche, and consultancy fees from GSK, Roche, Merck, Takeda, Exevir, and Allovir. Atul Humar has received clinical trial grants from Merck and consultancy fees from Merck and Takeda. The other authors have no conflicts of interest to disclose.
Appendix A. Supplementary data Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajt.2024.03.011.
References
Arora, Kempf, Nehlmeier, Omicron sublineage BQ.1.1 resistance to monoclonal antibodies, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00733-2
Banjongjit, Lertussavavivat, Paitoonpong, The predictors for severe SARS-CoV-2 Omicron (B.1.1.529) and pre-Omicron variants infection among kidney transplant recipients, Transplantation, doi:10.1097/TP.0000000000004361
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19final [report, N Engl J Med, doi:10.1056/NEJMoa2007764
Brenner, Ungaro, Gearry, Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry, Gastroenterology, doi:10.1053/j.gastro.2020.05.032
Chen, Luo, Mei, Immunogenicity of COVID-19 vaccines in solid organ transplant recipients: a systematic review and metaanalysis, Clin Microbiol Infect, doi:10.1016/j.cmi.2022.12.004
Clarke, Wiemken, Korenblat, Excess mortality among solid organ transplant recipients in the United States during the COVID-19 pandemic, Transplantation, doi:10.1097/TP.0000000000004341
Dauriat, Beaumont, Nguyen, Efficacy of three COVID-19 vaccine doses in lung transplant recipients: a multicentre cohort study, Eur Respir J, doi:10.1183/13993003.00502-2022
Fishbane, Hirsch, Nair, Special considerations for Paxlovid treatment among transplant recipients with SARS-CoV-2 infection, Am J Kidney Dis, doi:10.1053/j.ajkd.2022.01.001
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Heldman, Kates, Fisher, Limaye, Immunosuppression in solid organ transplant recipients with Covid-19: more data, but still complicated, Transpl Infect Dis, doi:10.1111/tid.13650
Heldman, Kates, Safa, Changing trends in mortality among solid organ transplant recipients hospitalized for COVID-19 during the course of the pandemic, Am J Transplant, doi:10.1111/ajt.16840
Jurdi, Morena, Cote, Bethea, Azzi et al., Tixagevimab/ cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave, Am J Transplant, doi:10.1111/ajt.17128
Kaminski, Gigan, Vermorel, COVID-19 morbidity decreases with tixagevimab-cilgavimab preexposure prophylaxis in kidney transplant recipient nonresponders or low-vaccine responders, Kidney Int, doi:10.1016/j.kint.2022.07.008
Kates, Haydel, Florman, Coronavirus disease 2019 in solid organ transplant: a multicenter cohort study, Clin Infect Dis, doi:10.1093/cid/ciaa1097
Levin, Ustianowski, Wit, Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of Covid-19, N Engl J Med, doi:10.1056/NEJMoa2116620
Linares, Cofan, Diekmann, A propensity score-matched analysis of mortality in solid organ transplant patients with COVID-19 compared to non-solid organ transplant patients, PLoS One, doi:10.1371/journal.pone.0247251
Malahe, Hoek, Dalm, Clinical characteristics and outcomes of immunocompromised patients with coronavirus disease 2019 caused by the omicron variant: A prospective, observational study, Clin Infect Dis, doi:10.1093/cid/ciac571
Overvad, Koch, Jespersen, Outcomes following SARS-CoV-2 infection in individuals with and without solid organ transplantation-a Danish nationwide cohort study, Am J Transplant, doi:10.1111/ajt.17142
Pereira, Mohan, Cohen, COVID-19 in solid organ transplant recipients: initial report from the US epicenter, Am J Transplant, doi:10.1111/ajt.15941
Planas, Bruel, Staropoli, Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888
Qu, Evans, Faraone, Distinct neutralizing antibody escape of SARS-CoV-2 Omicron subvariants BQ, BA, doi:10.1101/2022.10.19.512891
Solera, Arbol, Alshahrani, Impact of vaccination and early monoclonal antibody therapy on coronavirus disease 2019 outcomes in organ transplant recipients during the omicron wave, Clin Infect Dis, doi:10.1093/cid/ciac324
Solera, Arbol, Bahinskaya, Marks, Humar et al., Shortcourse early outpatient remdesivir prevents severe disease due to COVID-19 in organ transplant recipients during the omicron BA.2 wave, Am J Transplant, doi:10.1111/ajt.17199
Solera, Arbol, Ferreira, Differential serum neutralisation of omicron sublineages in patients receiving prophylaxis with tixagevimab-cilgavimab, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00208-6
Solera, Ierullo, Arbol, Bivalent COVID-19 mRNA vaccine against omicron subvariants in immunocompromised patients, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00357-2
Uraki, Ito, Furusawa, Humoral immune evasion of the omicron subvariants BQ.1.1 and XBB, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00816-7
Villanego, Vigara, Alonso, Trends in COVID-19 outcomes in kidney transplant recipients during the period of Omicron variant predominance, Transplantation, doi:10.1097/TP.0000000000004126
Wang, Iketani, Li, Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants, Cell, doi:10.1016/j.cell.2022.12.018
Weiss, Hendrickx, Stensgaard, Jellingsø, Sommer, Kidney transplant and dialysis patients remain at increased risk for succumbing to COVID-19, Transplantation, doi:10.1097/TP.0000000000004462
DOI record:
{
"DOI": "10.1016/j.ajt.2024.03.011",
"ISSN": [
"1600-6135"
],
"URL": "http://dx.doi.org/10.1016/j.ajt.2024.03.011",
"alternative-id": [
"S1600613524002077"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Longitudinal outcomes of COVID-19 in solid organ transplant recipients from 2020 to 2023"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "American Journal of Transplantation"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ajt.2024.03.011"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 The Author(s). Published by Elsevier Inc. on behalf of American Society of Transplantation & American Society of Transplant Surgeons."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6833-6625",
"affiliation": [],
"authenticated-orcid": false,
"family": "Solera",
"given": "Javier T.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-2880-3780",
"affiliation": [],
"authenticated-orcid": false,
"family": "Árbol",
"given": "Berta G.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7666-6234",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mittal",
"given": "Ankit",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9353-0059",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hall",
"given": "Victoria",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0404-787X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Marinelli",
"given": "Tina",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0004-4273-2207",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bahinskaya",
"given": "Ilona",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9435-2597",
"affiliation": [],
"authenticated-orcid": false,
"family": "Selzner",
"given": "Nazia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8026-9274",
"affiliation": [],
"authenticated-orcid": false,
"family": "McDonald",
"given": "Michael",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0493-0646",
"affiliation": [],
"authenticated-orcid": false,
"family": "Schiff",
"given": "Jeffrey",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4817-1533",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sidhu",
"given": "Aman",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1751-7159",
"affiliation": [],
"authenticated-orcid": false,
"family": "Humar",
"given": "Atul",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1961-0477",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kumar",
"given": "Deepali",
"sequence": "additional"
}
],
"container-title": [
"American Journal of Transplantation"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"amjtransplant.org",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
3,
17
]
],
"date-time": "2024-03-17T01:14:03Z",
"timestamp": 1710638043000
},
"deposited": {
"date-parts": [
[
2024,
4,
30
]
],
"date-time": "2024-04-30T12:37:47Z",
"timestamp": 1714480667000
},
"funder": [
{
"DOI": "10.13039/501100000014",
"doi-asserted-by": "publisher",
"name": "Canadian Blood Services"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
1
]
],
"date-time": "2024-05-01T00:21:36Z",
"timestamp": 1714522896149
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "1600-6135"
}
],
"issued": {
"date-parts": [
[
2024,
3
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T00:00:00Z",
"timestamp": 1709251200000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T00:00:00Z",
"timestamp": 1709251200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 14,
"start": {
"date-parts": [
[
2024,
3,
15
]
],
"date-time": "2024-03-15T00:00:00Z",
"timestamp": 1710460800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1600613524002077?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1600613524002077?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
3
]
]
},
"published-print": {
"date-parts": [
[
2024,
3
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1111/ajt.17142",
"article-title": "Outcomes following SARS-CoV-2 infection in individuals with and without solid organ transplantation—a Danish nationwide cohort study",
"author": "Overvad",
"doi-asserted-by": "crossref",
"first-page": "2627",
"issue": "11",
"journal-title": "Am J Transplant",
"key": "10.1016/j.ajt.2024.03.011_bib1",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1097/TP.0000000000004341",
"article-title": "Excess mortality among solid organ transplant recipients in the United States during the COVID-19 pandemic",
"author": "Clarke",
"doi-asserted-by": "crossref",
"first-page": "2399",
"issue": "12",
"journal-title": "Transplantation",
"key": "10.1016/j.ajt.2024.03.011_bib2",
"volume": "106",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciaa1097",
"article-title": "Coronavirus disease 2019 in solid organ transplant: a multicenter cohort study",
"author": "Kates",
"doi-asserted-by": "crossref",
"first-page": "e4090",
"issue": "11",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ajt.2024.03.011_bib3",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0247251",
"article-title": "A propensity score-matched analysis of mortality in solid organ transplant patients with COVID-19 compared to non-solid organ transplant patients",
"author": "Linares",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "PLoS One",
"key": "10.1016/j.ajt.2024.03.011_bib4",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1097/TP.0000000000004462",
"article-title": "Kidney transplant and dialysis patients remain at increased risk for succumbing to COVID-19",
"author": "Weiss",
"doi-asserted-by": "crossref",
"first-page": "1136",
"issue": "5",
"journal-title": "Transplantation",
"key": "10.1016/j.ajt.2024.03.011_bib5",
"volume": "107",
"year": "2023"
},
{
"DOI": "10.1097/TP.0000000000004361",
"article-title": "The predictors for severe SARS-CoV-2 Omicron (B.1.1.529) and pre-Omicron variants infection among kidney transplant recipients",
"author": "Banjongjit",
"doi-asserted-by": "crossref",
"first-page": "e520",
"issue": "12",
"journal-title": "Transplantation",
"key": "10.1016/j.ajt.2024.03.011_bib6",
"volume": "106",
"year": "2022"
},
{
"DOI": "10.1053/j.gastro.2020.05.032",
"article-title": "Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry",
"author": "Brenner",
"doi-asserted-by": "crossref",
"first-page": "481",
"issue": "2",
"journal-title": "Gastroenterology",
"key": "10.1016/j.ajt.2024.03.011_bib7",
"volume": "159",
"year": "2020"
},
{
"DOI": "10.1111/ajt.17199",
"article-title": "Short-course early outpatient remdesivir prevents severe disease due to COVID-19 in organ transplant recipients during the omicron BA.2 wave",
"author": "Solera",
"doi-asserted-by": "crossref",
"first-page": "78",
"issue": "1",
"journal-title": "Am J Transplant",
"key": "10.1016/j.ajt.2024.03.011_bib8",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(23)00357-2",
"article-title": "Bivalent COVID-19 mRNA vaccine against omicron subvariants in immunocompromised patients",
"author": "Solera",
"doi-asserted-by": "crossref",
"first-page": "e266",
"issue": "8",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.ajt.2024.03.011_bib9",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(22)00816-7",
"article-title": "Humoral immune evasion of the omicron subvariants BQ.1.1 and XBB",
"author": "Uraki",
"doi-asserted-by": "crossref",
"first-page": "30",
"issue": "1",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.ajt.2024.03.011_bib10",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2116620",
"article-title": "Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of Covid-19",
"author": "Levin",
"doi-asserted-by": "crossref",
"first-page": "2188",
"issue": "23",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ajt.2024.03.011_bib11",
"volume": "386",
"year": "2022"
},
{
"article-title": "Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies",
"author": "Planas",
"journal-title": "bioRxiv",
"key": "10.1016/j.ajt.2024.03.011_bib12",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(23)00208-6",
"article-title": "Differential serum neutralisation of omicron sublineages in patients receiving prophylaxis with tixagevimab-cilgavimab",
"author": "Solera",
"doi-asserted-by": "crossref",
"first-page": "528",
"issue": "5",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.ajt.2024.03.011_bib13",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/j.cell.2022.12.018",
"article-title": "Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "279",
"issue": "2",
"journal-title": "Cell",
"key": "10.1016/j.ajt.2024.03.011_bib14",
"volume": "186",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(22)00733-2",
"article-title": "Omicron sublineage BQ.1.1 resistance to monoclonal antibodies",
"author": "Arora",
"doi-asserted-by": "crossref",
"first-page": "22",
"issue": "1",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.ajt.2024.03.011_bib15",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19 – final [report]",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ajt.2024.03.011_bib16",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ajt.2024.03.011_bib17",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1053/j.ajkd.2022.01.001",
"article-title": "Special considerations for Paxlovid treatment among transplant recipients with SARS-CoV-2 infection",
"author": "Fishbane",
"doi-asserted-by": "crossref",
"first-page": "480",
"issue": "4",
"journal-title": "Am J Kidney Dis",
"key": "10.1016/j.ajt.2024.03.011_bib18",
"volume": "79",
"year": "2022"
},
{
"key": "10.1016/j.ajt.2024.03.011_bib19",
"series-title": "Antiviral and Antibody Products Summary Recommendations",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac324",
"article-title": "Impact of vaccination and early monoclonal antibody therapy on coronavirus disease 2019 outcomes in organ transplant recipients during the omicron wave",
"author": "Solera",
"doi-asserted-by": "crossref",
"first-page": "2193",
"issue": "12",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ajt.2024.03.011_bib20",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac571",
"article-title": "Clinical characteristics and outcomes of immunocompromised patients with coronavirus disease 2019 caused by the omicron variant: A prospective, observational study",
"author": "Malahe",
"doi-asserted-by": "crossref",
"first-page": "e172",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ajt.2024.03.011_bib21",
"volume": "76",
"year": "2023"
},
{
"key": "10.1016/j.ajt.2024.03.011_bib22",
"series-title": "SARS-CoV-2 Genomic Surveillance in Ontario, March 24, 2023",
"year": "2023"
},
{
"DOI": "10.1097/TP.0000000000004126",
"article-title": "Trends in COVID-19 outcomes in kidney transplant recipients during the period of Omicron variant predominance",
"author": "Villanego",
"doi-asserted-by": "crossref",
"first-page": "e304",
"issue": "6",
"journal-title": "Transplantation",
"key": "10.1016/j.ajt.2024.03.011_bib24",
"volume": "106",
"year": "2022"
},
{
"article-title": "Distinct neutralizing antibody escape of SARS-CoV-2 Omicron subvariants BQ.1, BQ.1.1, BA.4.6, BF.7 and BA.2.75.2",
"author": "Qu",
"journal-title": "bioRxiv",
"key": "10.1016/j.ajt.2024.03.011_bib25",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2022.12.004",
"article-title": "Immunogenicity of COVID-19 vaccines in solid organ transplant recipients: a systematic review and meta-analysis",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "441",
"issue": "4",
"journal-title": "Clin Microbiol Infect",
"key": "10.1016/j.ajt.2024.03.011_bib26",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1183/13993003.00502-2022",
"article-title": "Efficacy of three COVID-19 vaccine doses in lung transplant recipients: a multicentre cohort study",
"author": "Dauriat",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Eur Respir J",
"key": "10.1016/j.ajt.2024.03.011_bib27",
"volume": "61",
"year": "2023"
},
{
"DOI": "10.1111/ajt.15941",
"article-title": "COVID-19 in solid organ transplant recipients: initial report from the US epicenter",
"author": "Pereira",
"doi-asserted-by": "crossref",
"first-page": "1800",
"issue": "7",
"journal-title": "Am J Transplant",
"key": "10.1016/j.ajt.2024.03.011_bib28",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1111/tid.13650",
"article-title": "Immunosuppression in solid organ transplant recipients with Covid-19: more data, but still complicated",
"author": "Heldman",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "Transpl Infect Dis",
"key": "10.1016/j.ajt.2024.03.011_bib29",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1111/ajt.16840",
"article-title": "Changing trends in mortality among solid organ transplant recipients hospitalized for COVID-19 during the course of the pandemic",
"author": "Heldman",
"doi-asserted-by": "crossref",
"first-page": "279",
"issue": "1",
"journal-title": "Am J Transplant",
"key": "10.1016/j.ajt.2024.03.011_bib30",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1111/ajt.17128",
"article-title": "Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave",
"author": "Al Jurdi",
"doi-asserted-by": "crossref",
"first-page": "3130",
"issue": "12",
"journal-title": "Am J Transplant",
"key": "10.1016/j.ajt.2024.03.011_bib31",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/j.kint.2022.07.008",
"article-title": "COVID-19 morbidity decreases with tixagevimab-cilgavimab preexposure prophylaxis in kidney transplant recipient nonresponders or low-vaccine responders",
"author": "Kaminski",
"doi-asserted-by": "crossref",
"first-page": "936",
"issue": "4",
"journal-title": "Kidney Int",
"key": "10.1016/j.ajt.2024.03.011_bib32",
"volume": "102",
"year": "2022"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1600613524002077"
}
},
"score": 1,
"short-container-title": [
"American Journal of Transplantation"
],
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": [
"Longitudinal outcomes of COVID-19 in solid organ transplant recipients from 2020 to 2023"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}