Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave
et al., American Journal of Transplantation, doi:10.1111/ajt.17128, Dec 2022
42nd treatment shown to reduce risk in
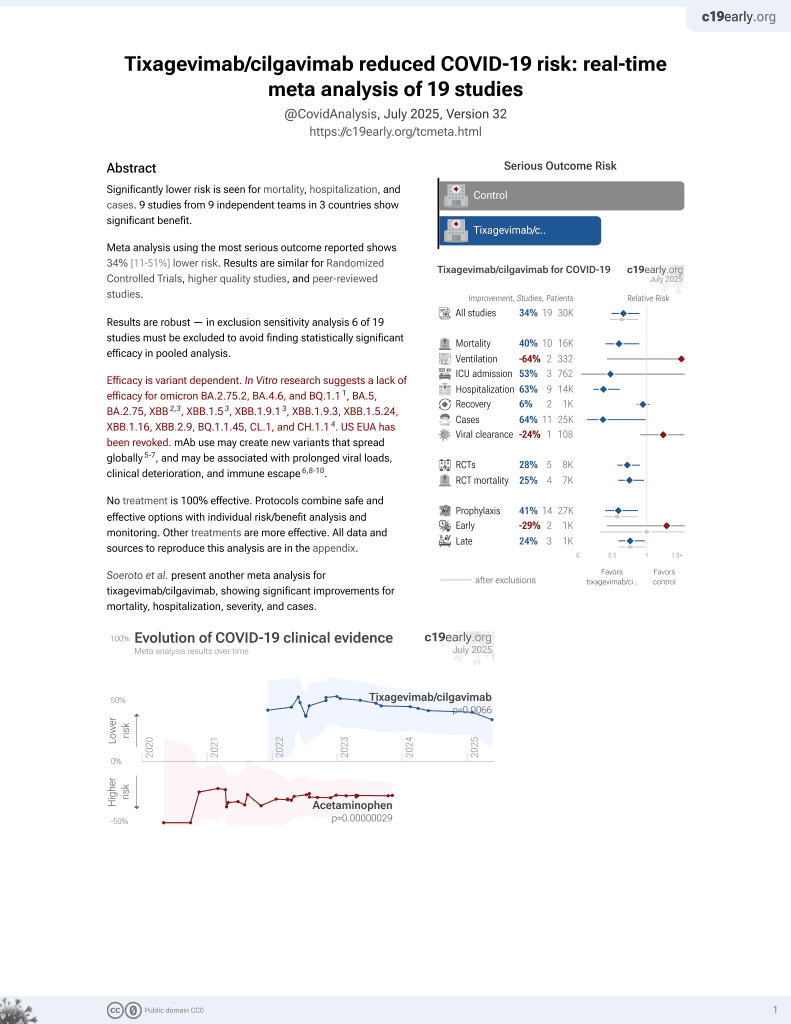
May 2022, now with p = 0.0066 from 19 studies, recognized in 33 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective cohort study of 444 solid organ transplant recipients showing significantly lower risk of SARS-CoV-2 breakthrough infections with tixagevimab/cilgavimab pre-exposure prophylaxis compared to controls during the omicron period.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending tixagevimab/cilgavimab also recommended them, or
because the patient seeking out tixagevimab/cilgavimab is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Therefore, these kind of studies may
overestimate efficacy.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2.75.2, BA.4.6, BQ.1.14, BA.5, BA.2.75, XBB5,6, XBB.1.56, ХВВ.1.9.16, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.17.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 85.7% lower, RR 0.14, p = 0.25, treatment 0 of 222 (0.0%), control 3 of 222 (1.4%), NNT 74, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 83.3% lower, RR 0.17, p = 0.12, treatment 1 of 222 (0.5%), control 6 of 222 (2.7%), NNT 44.
|
|
risk of case, 65.6% lower, RR 0.34, p = 0.001, treatment 11 of 222 (5.0%), control 32 of 222 (14.4%), NNT 11.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
5.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
6.
Uraki et al., Antiviral efficacy against and replicative fitness of an XBB.1.9.1 clinical isolate, iScience, doi:10.1016/j.isci.2023.108147.
Al Jurdi et al., 31 Dec 2022, retrospective, USA, peer-reviewed, 6 authors.
Contact: lriella@mgh.harvard.edu.
Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave
American Journal of Transplantation, doi:10.1111/ajt.17128
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in solid organ transplant recipients (SOTRs) is associated with higher mortality compared to immunocompetent individuals. 1 Since antiviral responses to SARS-CoV-2 vaccines in SOTRs are attenuated, 2 additional strategies such as monoclonal antibody pre-exposure prophylaxis have been developed. 3 Tixagevimab and cilgavimab are neutralizing monoclonal antibodies directed against different epitopes of the receptor-binding domain of SARS-CoV-2 spike protein that have been associated with a lower risk of SARS-CoV-2 infection when used for pre-exposure prophylaxis in unvaccinated individuals. 4 Based on that, tixagevimab/cilgavimab received emergency use authorization from the US Food and Drug
| 3135 AJT AL JURDI et AL. We also found that tixagevimab/cilgavimab use was associated with a lower incidence of breakthrough infection regardless of whether SOTRs had received 0-3 or 4-5 vaccines doses. Separate analysis of SOTRs who received 0-2 or 5 vaccine doses was not performed due to the small number of SOTRs in these groups. In addition, we found that tixagevimab/cilgavimab use was associated with a lower risk of breakthrough infection in kidney and lung transplant recipients. There was no statistically significant difference in the risk of breakthrough infection between liver or liver/kidney transplant recipients who did and did not receive tixagevimab/cilgavimab. However, given the small number of liver and liver/kidney transplant recipients in our cohort, our study was underpowered to find differences in this subgroup of SOTRs. Importantly, we also found a higher incidence of breakthrough infections in the low-dose (150-150 mg) compared to the high-dose (300-300 mg) tixagevimab/cilgavimab cohort, suggesting a lower risk of breakthrough infections in the high-dose group, which supports the FDA's revised recommendation to use the higher dose for preexposure prophylaxis in SOTRs. 14 A randomized controlled trial comparing the two doses would provide further evidence supporting the difference in breakthrough infection between the two doses in SOTRs. We also found that tixagevimab/cilgavimab was safe with a low rate of adverse events and no evidence of allograft..
References
Altarawneh, Chemaitelly, Hasan, Protection against the omicron variant from previous SARS-CoV-2 infection, N Engl J Med, doi:10.1056/NEJMc2200133
Andrews, Stowe, Kirsebom, Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant, N Engl J Med, doi:10.1056/NEJMoa2119451
Bertrand, Laurent, Lemée, Efficacy of anti SARS-CoV-2 monoclonal antibodies prophylaxis and vaccination on omicron COVID-19 in kidney transplant recipients, Kidney Int, doi:10.1016/j.kint.2022.05.007
Caillard, Chavarot, Francois, Is COVID-19 infection more severe in kidney transplant recipients?, Am J Transplant, doi:10.1111/ajt.16424
Goldberg, Mandel, Bar-On, Protection and waning of natural and hybrid immunity to SARS-CoV-2, N Engl J Med, doi:10.1056/NEJMoa2118946
Hall, Foulkes, Charlett, SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN), Lancet, doi:10.1016/S0140-6736(21)00675-9
Hansen, Michlmayr, Gubbels, Mølbak, Ethelberg, Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study, Lancet, doi:10.1016/S0140-6736(21)00575-4
Jurdi, Gassen, Borges, Non-invasive monitoring for rejection in kidney transplant recipients after SARS-CoV-2 mRNA vaccination, Front Immunol, doi:10.3389/fimmu.2022.838985
Levin, Ustianowski, Wit, Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of Covid-19, N Engl J Med, doi:10.1056/NEJMoa2116620
Loo, Mctamney, Arends, The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans, Sci Transl Med, doi:10.1126/scitranslmed.abl8124
Magen, Waxman, Makov-Assif, Fourth dose of BNT162b2 mRNA Covid-19 vaccine in a Nationwide setting, N Engl J Med, doi:10.1056/NEJMoa2201688
Sheehan, Reddy, Rothberg, Reinfection rates among patients who previously tested positive for coronavirus disease 2019: a retrospective cohort study, Clin Infect Dis, doi:10.1093/cid/ciab234
DOI record:
{
"DOI": "10.1111/ajt.17128",
"ISSN": [
"1600-6135"
],
"URL": "http://dx.doi.org/10.1111/ajt.17128",
"alternative-id": [
"S1600613523000655"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "American Journal of Transplantation"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1111/ajt.17128"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "Copyright © 2022 American Society of Transplantation & American Society of Transplant Surgeons. Published by Elsevier Inc. All rights reserved. Published by Elsevier B.V. All rights reserved."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7652-3399",
"affiliation": [],
"authenticated-orcid": false,
"family": "Al Jurdi",
"given": "Ayman",
"sequence": "first"
},
{
"affiliation": [],
"family": "Morena",
"given": "Leela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cote",
"given": "Mariesa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bethea",
"given": "Emily",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6909-4645",
"affiliation": [],
"authenticated-orcid": false,
"family": "Azzi",
"given": "Jamil",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7636-3196",
"affiliation": [],
"authenticated-orcid": false,
"family": "Riella",
"given": "Leonardo V.",
"sequence": "additional"
}
],
"container-title": "American Journal of Transplantation",
"container-title-short": "American Journal of Transplantation",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"amjtransplant.org",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
6,
21
]
],
"date-time": "2022-06-21T18:18:34Z",
"timestamp": 1655835514000
},
"deposited": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T12:55:33Z",
"timestamp": 1701435333000
},
"funder": [
{
"DOI": "10.13039/100005294",
"award": [
"Harold and Ellen Danser Endowed/Distinguished Chai"
],
"doi-asserted-by": "publisher",
"name": "Massachusetts General Hospital"
}
],
"indexed": {
"date-parts": [
[
2024,
1,
6
]
],
"date-time": "2024-01-06T22:57:33Z",
"timestamp": 1704581853900
},
"is-referenced-by-count": 70,
"issue": "12",
"issued": {
"date-parts": [
[
2022,
12
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 365,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/ajt.17128",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/ajt.17128",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1600613523000655?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1600613523000655?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/ajt.17128",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "78",
"original-title": [],
"page": "3130-3136",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
12
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1111/ajt.16424",
"article-title": "Is COVID-19 infection more severe in kidney transplant recipients?",
"author": "Caillard",
"doi-asserted-by": "crossref",
"first-page": "1295",
"issue": "3",
"journal-title": "Am J Transplant.",
"key": "10.1111/ajt.17128_bib1",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2022.838985",
"article-title": "Non-invasive monitoring for rejection in kidney transplant recipients after SARS-CoV-2 mRNA vaccination",
"author": "Al Jurdi",
"doi-asserted-by": "crossref",
"first-page": "838985",
"journal-title": "Front Immunol.",
"key": "10.1111/ajt.17128_bib2",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1126/scitranslmed.abl8124",
"article-title": "The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans",
"author": "Loo",
"doi-asserted-by": "crossref",
"first-page": "eabl8124",
"issue": "635",
"journal-title": "Sci Transl Med.",
"key": "10.1111/ajt.17128_bib3",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116620",
"article-title": "Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of Covid-19",
"author": "Levin",
"doi-asserted-by": "crossref",
"first-page": "2188",
"journal-title": "N Engl J Med.",
"key": "10.1111/ajt.17128_bib4",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1001/jama.2021.24931",
"article-title": "Tixagevimab and Cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19",
"doi-asserted-by": "crossref",
"first-page": "384",
"issue": "4",
"journal-title": "Jama.",
"key": "10.1111/ajt.17128_bib5",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2201688",
"article-title": "Fourth dose of BNT162b2 mRNA Covid-19 vaccine in a Nationwide setting",
"author": "Magen",
"doi-asserted-by": "crossref",
"first-page": "1603",
"issue": "17",
"journal-title": "N Engl J Med.",
"key": "10.1111/ajt.17128_bib6",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2119451",
"article-title": "Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant",
"author": "Andrews",
"doi-asserted-by": "crossref",
"first-page": "1532",
"issue": "16",
"journal-title": "N Engl J Med.",
"key": "10.1111/ajt.17128_bib7",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2200133",
"article-title": "Protection against the omicron variant from previous SARS-CoV-2 infection",
"author": "Altarawneh",
"doi-asserted-by": "crossref",
"first-page": "1288",
"issue": "13",
"journal-title": "N Engl J Med.",
"key": "10.1111/ajt.17128_bib8",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118946",
"article-title": "Protection and waning of natural and hybrid immunity to SARS-CoV-2",
"author": "Goldberg",
"doi-asserted-by": "crossref",
"first-page": "2201",
"journal-title": "N Engl J Med.",
"key": "10.1111/ajt.17128_bib9",
"volume": "25",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(21)00675-9",
"article-title": "SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN)",
"author": "Hall",
"doi-asserted-by": "crossref",
"first-page": "1459",
"issue": "10283",
"journal-title": "Lancet (London, England).",
"key": "10.1111/ajt.17128_bib10",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00575-4",
"article-title": "Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study",
"author": "Hansen",
"doi-asserted-by": "crossref",
"first-page": "1204",
"issue": "10280",
"journal-title": "Lancet (London, England).",
"key": "10.1111/ajt.17128_bib11",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciab234",
"article-title": "Reinfection rates among patients who previously tested positive for coronavirus disease 2019: a retrospective cohort study",
"author": "Sheehan",
"doi-asserted-by": "crossref",
"first-page": "1882",
"issue": "10",
"journal-title": "Clin Infect Dis.",
"key": "10.1111/ajt.17128_bib12",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1016/j.kint.2022.05.007",
"article-title": "Efficacy of anti SARS-CoV-2 monoclonal antibodies prophylaxis and vaccination on omicron COVID-19 in kidney transplant recipients",
"author": "Bertrand",
"doi-asserted-by": "crossref",
"journal-title": "Kidney Int.",
"key": "10.1111/ajt.17128_bib13",
"year": "2022"
},
{
"key": "10.1111/ajt.17128_bib14",
"unstructured": "FDA. FDA authorizes revisions to Evusheld dosing. 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-revisions-evusheld-dosing"
},
{
"key": "10.1111/ajt.17128_bib15",
"unstructured": "CDC: Variant proportions. 2022. Accessed May 1, 2022. https://covid.cdc.gov/covid-data-tracker/#variant-proportions"
}
],
"reference-count": 15,
"references-count": 15,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1600613523000655"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Transplantation",
"Immunology and Allergy"
],
"subtitle": [],
"title": "Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "22"
}