Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies
et al., bioRxiv, doi:10.1101/2022.11.17.516888, Nov 2022
42nd treatment shown to reduce risk in
May 2022, now with p = 0.0066 from 19 studies, recognized in 33 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro study suggesting a lack of efficacy for tixagevimab/vilgavimab with BA.2.75.2, BQ.1.1, and BA.4.6.
Study covers bebtelovimab, casirivimab/imdevimab, and tixagevimab/cilgavimab.
Planas et al., 17 Nov 2022, preprint, 25 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies
doi:10.1101/2022.11.17.516888
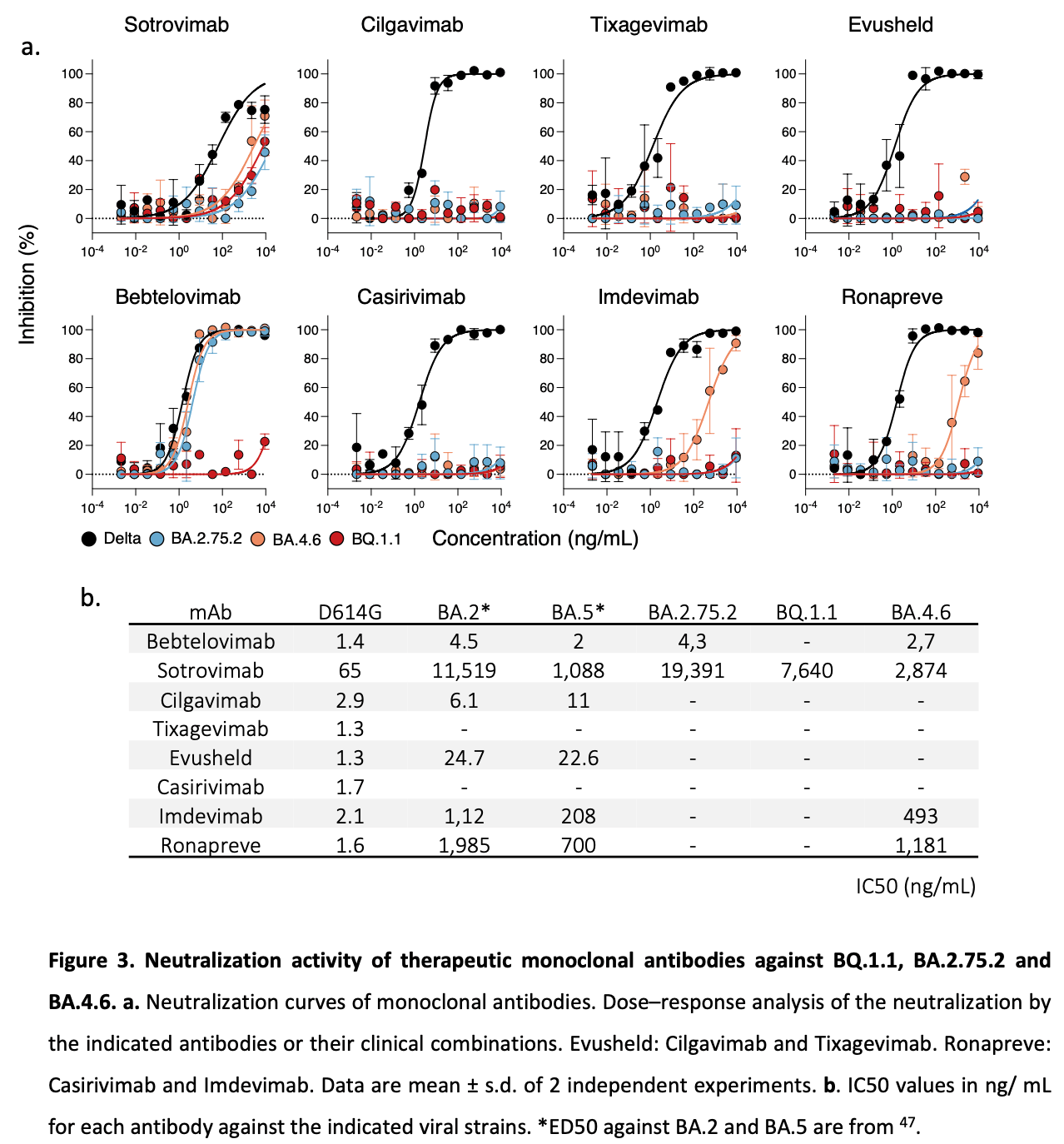
Convergent evolution of SARS-CoV-2 Omicron BA.2, BA.4 and BA.5 lineages has led to the emergence of several new subvariants, including BA.2.75.2, BA.4.6. and BQ.1.1. The subvariants BA.2.75.2 and BQ.1.1 are expected to become predominant in many countries in November 2022. They carry an additional and often redundant set of mutations in the spike, likely responsible for increased transmissibility and immune evasion. Here, we established a viral amplification procedure to easily isolate Omicron strains. We examined their sensitivity to 6 therapeutic monoclonal antibodies (mAbs) and to 72 sera from Pfizer BNT162b2-vaccinated individuals, with or without BA.1/BA.2 or BA.5 breakthrough infection. Ronapreve (Casirivimab and Imdevimab) and Evusheld (Cilgavimab and Tixagevimab) lost any antiviral efficacy against BA.2.75.2 and BQ.1.1, whereas Xevudy (Sotrovimab) remained weakly active. BQ.1.1 was also resistant to Bebtelovimab. Neutralizing titers in triply vaccinated individuals were low to undetectable against BQ.1.1 and BA.2.75.2, 4 months after boosting. A BA.1/BA.2 breakthrough infection increased these titers, which remained about 18-fold lower against BA.2.75.2 and BQ.1.1, than against BA.1. Reciprocally, a BA.5 breakthrough infection increased more efficiently neutralization against BA.5 and BQ.1.1 than against BA.2.75.2. Thus, the evolution trajectory of novel Omicron subvariants facilitated their spread in immunized populations and raises concerns about the efficacy of most currently available mAbs. .
Vital materials: P.M., C.P., J.P., M.S., R.S., F.F., N.M., J.D., R.S., H.M., E.A., L.H., D.V., T.P., H.P. Viral sequencing: P.M., M.P., S.M., J.P., E.S-L., D.V., H.P. Manuscript writing and editing: D.P., T.B., O.S.
Methods No statistical methods were used to predetermine sample size and the experiments were not randomized. The investigators were not blinded. Our research fulfills all relevant ethical requirements.
Cohorts Serum from vaccinated and BA.1/2 and BA.5 breakthrough infected individuals (Orléans cohort). A prospective, monocentric, longitudinal, interventional cohort clinical study (ABCOVID) is conducted since 27 August 2020 with the objective to study the kinetics of COVID-19 antibodies in patients with confirmed SARS-CoV-2 infection (NCT04750720). A sub-study aimed to describe the kinetic of neutralizing antibodies after vaccination. The cohort was previously described 11, 16 . This study was approved by the Ile-de-France IV ethical committee. At enrollment, written informed consent was collected and participants completed a questionnaire covering sociodemographic characteristics. Virological findings (SARS-CoV-2 RT-qPCR results, date of positive test, screening, or sequences results) and data related to SARS-CoV-2 vaccination (brand product, date of first, second, third and fourth vaccination) were also collected. Nasopharyngeal swabs from infected individuals (Hôpital Européen Georges Pompidou). 134 nasopharyngeal swabs collected for standard care between..
References
Abu-Raddad, Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar, New England Journal of Medicine
Andrews, Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant, New England Journal of Medicine
Arora, Lung cell entry, cell-cell fusion capacity, and neutralisation sensitivity of omicron sublineage BA.2.75, The Lancet Infectious Diseases
Bruel, Longitudinal analysis of serum neutralization of SARS-CoV-2 Omicron BA.2, BA.4 and BA.5 in patients receiving monoclonal antibodies, medRxiv
Bruel, Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies, Nature Medicine
Buchrieser, Syncytia formation by SARS-CoV-2-infected cells, EMBO J
Buckner, Interval between prior SARS-CoV-2 infection and booster vaccination impacts magnitude and quality of antibody and B cell responses, Cell
Bénard, Characterization of a human ovarian adenocarcinoma line, IGROV1, in tissue culture and in nude mice, Cancer Res
Cao, 2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection, Nature
Cao, 4 and BA.5 escape antibodies elicited by Omicron infection, Nature
Cao, Characterization of the enhanced infectivity and antibody evasion of Omicron BA.2.75, Cell Host & Microbe
Cao, Imprinted SARS-CoV-2 humoral immunity induces converging Omicron RBD evolution, Biorxiv
Cao, Rapid evaluation of COVID-19 vaccine effectiveness against symptomatic infection with SARS-CoV-2 variants by analysis of genetic distance, Nature Medicine
Carreño, Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron, Nature
Cele, Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization, Nature
Chalkias, Safety, immunogenicity and antibody persistence of a bivalent Betacontaining booster vaccine against COVID-19: a phase 2/3 trial, Nature Medicine
Collier, -R, Immunogenicity of the BA.5 Bivalent mRNA Vaccine Boosters, Biorxiv
Davis-Gardner, mRNA bivalent booster enhances neutralization against BA, BQ
France, Point épidémiologique COVID-19 du 10
Gruell, Antibody-mediated neutralization of SARS-CoV-2, Immunity
Gupta, Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab, New England Journal of Medicine
Hachmann, Miller, Collier, Barouch, Neutralization Escape by SARS-CoV-2
Hachmann, Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, New England Journal of Medicine
Hentzien, Autran, Piroth, Yazdanpanah, Calmy, A monoclonal antibody stands out against omicron subvariants: a call to action for a wider access to bebtelovimab, The Lancet Infectious Diseases
Kimura, Virological characteristics of the SARS-CoV-2 Omicron BA.2 subvariants, including BA.4 and BA.5, Cell
Kuhlmann, Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose, The Lancet
Kurhade, .1.1, and XBB.1 by 4 doses of parental mRNA vaccine or a BA.5-bivalent booster, Biorxiv
Lee, Wheatley, Kent, Dekosky, Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies, Nature Microbiology
Levin, Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19, New England Journal of Medicine
Martin-Blondel, Sotrovimab to prevent severe COVID-19 in high-risk patients infected with Omicron BA.2, J Infection
Mccallum, N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2, Cell
Meng, Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity, Nature
Miller, Substantial Neutralization Escape by the SARS-CoV-2 Omicron Variant BQ
Monel, Release of infectious virus and cytokines in nasopharyngeal swabs from individuals infected with non-alpha or alpha SARS-CoV-2 variants: an observational retrospective study, eBioMedicine
Murray, COVID-19 will continue but the end of the pandemic is near, The Lancet
Ng, SARS-CoV-2 S2–targeted vaccination elicits broadly neutralizing antibodies, Science Translational Medicine
O'brien, Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19, New England Journal of Medicine
Ogando, SARS-coronavirus-2 replication in Vero E6 cells: replication kinetics, rapid adaptation and cytopathology, Journal of General Virology
Osada, The genome landscape of the african green monkey kidney-derived vero cell line, DNA Res
Park, Imprinted antibody responses against SARS-CoV-2 Omicron sublineages, Science
Park, Imprinted antibody responses against SARS-CoV-2 Omicron sublineages, Science
Planas, Considerable escape of SARS-CoV-2 Omicron to antibody neutralization, Nature
Planas, Duration of BA.5 neutralization in sera and nasal swabs from SARS-CoV-2 vaccinated individuals, with or without omicron breakthrough infection, Med
Planas, Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies, Nature Medicine
Puhach, Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2, Nature Medicine
Qu, Distinct Neutralizing Antibody Escape of SARS-CoV-2 Omicron Subvariants BQ, BF.7 and BA
Reynolds, Immune boosting by B.1.1.529 <b>(</b>Omicron) depends on previous SARS-CoV-2 exposure, Science
Saito, Virological characteristics of the SARS-CoV-2 Omicron BA.2.75 variant, Cell Host & Microbe
Scheaffer, Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant in mice, Nature Medicine
Sheward, .2.75.2 exhibits extensive escape from neutralising antibodies, Omicron sublineage BA
Sun, SARS-CoV-2 transmission, persistence of immunity, and estimates of Omicron’s impact in South African population cohorts, Science Translational Medicine
Suzuki, Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant, Nature
Taylor, Neutralizing monoclonal antibodies for treatment of COVID-19, Nature Reviews Immunology
Tegally, Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa, Nature Medicine
Tuekprakhon, Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum, Cell
Viana, Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa, Nature
Walls, SARS-CoV-2 breakthrough infections elicit potent, broad and durable neutralizing antibody responses, Cell
Wang, Antibody responses to Omicron BA.4/BA.5 bivalent mRNA vaccine booster shot, Biorxiv
Wang, Resistance of SARS-CoV-2 omicron subvariant BA.4.6 to antibody neutralisation, The Lancet Infectious Diseases
Westendorf, LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants, Cell Reports
Who, Therapeutics and COVID-19: living guideline
Wu, WHO's Therapeutics and COVID-19 Living Guideline on mAbs needs to be reassessed, The Lancet
DOI record:
{
"DOI": "10.1101/2022.11.17.516888",
"URL": "http://dx.doi.org/10.1101/2022.11.17.516888",
"abstract": "<jats:p>Convergent evolution of SARS-CoV-2 Omicron BA.2, BA.4 and BA.5 lineages has led to the emergence of several new subvariants, including BA.2.75.2, BA.4.6. and BQ.1.1. The subvariants BA.2.75.2 and BQ.1.1 are expected to become predominant in many countries in November 2022. They carry an additional and often redundant set of mutations in the spike, likely responsible for increased transmissibility and immune evasion. Here, we established a viral amplification procedure to easily isolate Omicron strains. We examined their sensitivity to 6 therapeutic monoclonal antibodies (mAbs) and to 72 sera from Pfizer BNT162b2-vaccinated individuals, with or without BA.1/BA.2 or BA.5 breakthrough infection. Ronapreve (Casirivimab and Imdevimab) and Evusheld (Cilgavimab and Tixagevimab) lost any antiviral efficacy against BA.2.75.2 and BQ.1.1, whereas Xevudy (Sotrovimab) remained weakly active. BQ.1.1 was also resistant to Bebtelovimab. Neutralizing titers in triply vaccinated individuals were low to undetectable against BQ.1.1 and BA.2.75.2, 4 months after boosting. A BA.1/BA.2 breakthrough infection increased these titers, which remained about 18-fold lower against BA.2.75.2 and BQ.1.1, than against BA.1. Reciprocally, a BA.5 breakthrough infection increased more efficiently neutralization against BA.5 and BQ.1.1 than against BA.2.75.2. Thus, the evolution trajectory of novel Omicron subvariants facilitated their spread in immunized populations and raises concerns about the efficacy of most currently available mAbs.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
11,
21
]
]
},
"author": [
{
"affiliation": [],
"family": "Planas",
"given": "Delphine",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-3952-4261",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bruel",
"given": "Timothee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Staropoli",
"given": "Isabelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guivel-Benhassine",
"given": "Florence",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Porrot",
"given": "Francoise",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maes",
"given": "Piet",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1298-7565",
"affiliation": [],
"authenticated-orcid": false,
"family": "Grzelak",
"given": "Ludivine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prot",
"given": "Matthieu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mougari",
"given": "Said",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Planchais",
"given": "Cyril",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Puech",
"given": "Julien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saliba",
"given": "Madelina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sahraoui",
"given": "Riwan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Femy",
"given": "Florent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morel",
"given": "Nathalie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dufloo",
"given": "Jeremy",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1844-545X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sanjuan",
"given": "Rafael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mouquet",
"given": "Hugo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andre",
"given": "Emmanuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hocqueloux",
"given": "Laurent",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8420-7743",
"affiliation": [],
"authenticated-orcid": false,
"family": "Simon-Loriere",
"given": "Etienne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Veyer",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prazuck",
"given": "Thierry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pere",
"given": "Helene",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0729-1475",
"affiliation": [],
"authenticated-orcid": false,
"family": "Schwartz",
"given": "Olivier",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
11,
17
]
],
"date-time": "2022-11-17T21:35:16Z",
"timestamp": 1668720916000
},
"deposited": {
"date-parts": [
[
2022,
11,
21
]
],
"date-time": "2022-11-21T17:05:11Z",
"timestamp": 1669050311000
},
"group-title": "Microbiology",
"indexed": {
"date-parts": [
[
2022,
11,
22
]
],
"date-time": "2022-11-22T05:55:25Z",
"timestamp": 1669096525819
},
"institution": [
{
"name": "bioRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
11,
17
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.11.17.516888",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
11,
17
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
11,
17
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1038/s41586-022-04411-y",
"article-title": "Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa",
"doi-asserted-by": "crossref",
"first-page": "679",
"journal-title": "Nature",
"key": "2022111910400671000_2022.11.17.516888v1.1",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1038/s41591-022-01911-2",
"article-title": "Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa",
"doi-asserted-by": "crossref",
"first-page": "1785",
"journal-title": "Nature Medicine",
"key": "2022111910400671000_2022.11.17.516888v1.2",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00100-3",
"article-title": "COVID-19 will continue but the end of the pandemic is near",
"doi-asserted-by": "crossref",
"first-page": "417",
"journal-title": "The Lancet",
"key": "2022111910400671000_2022.11.17.516888v1.3",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1126/scitranslmed.abo7081",
"article-title": "SARS-CoV-2 transmission, persistence of immunity, and estimates of Omicron’s impact in South African population cohorts",
"doi-asserted-by": "crossref",
"first-page": "eabo7081",
"journal-title": "Science Translational Medicine",
"key": "2022111910400671000_2022.11.17.516888v1.4",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1056/nejmoa2119451",
"doi-asserted-by": "publisher",
"key": "2022111910400671000_2022.11.17.516888v1.5"
},
{
"DOI": "10.1056/NEJMoa2200797",
"article-title": "Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar",
"doi-asserted-by": "crossref",
"first-page": "1804",
"journal-title": "New England Journal of Medicine",
"key": "2022111910400671000_2022.11.17.516888v1.6",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00090-3",
"article-title": "Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose",
"doi-asserted-by": "crossref",
"first-page": "625",
"journal-title": "The Lancet",
"key": "2022111910400671000_2022.11.17.516888v1.7",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1101/2021.12.08.471707",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.8",
"unstructured": "Walls, A.C. , et al. SARS-CoV-2 breakthrough infections elicit potent, broad and durable neutralizing antibody responses. Cell (2022)."
},
{
"DOI": "10.1038/d41586-021-03824-5",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.9",
"unstructured": "Cele, S. , et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature (2021)."
},
{
"DOI": "10.1038/d41586-021-03846-z",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.10",
"unstructured": "Carreño, J.M. , et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature (2021)."
},
{
"DOI": "10.1038/s41586-021-04389-z",
"article-title": "Considerable escape of SARS-CoV-2 Omicron to antibody neutralization",
"doi-asserted-by": "crossref",
"first-page": "671",
"journal-title": "Nature",
"key": "2022111910400671000_2022.11.17.516888v1.11",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04980-y",
"article-title": "BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection",
"doi-asserted-by": "crossref",
"first-page": "593",
"journal-title": "Nature",
"key": "2022111910400671000_2022.11.17.516888v1.12",
"volume": "608",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2206576",
"article-title": "Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5",
"doi-asserted-by": "crossref",
"first-page": "86",
"journal-title": "New England Journal of Medicine",
"key": "2022111910400671000_2022.11.17.516888v1.13",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04980-y",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.14",
"unstructured": "Cao, Y. , et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature (2022)."
},
{
"DOI": "10.1016/j.cell.2022.06.005",
"doi-asserted-by": "publisher",
"key": "2022111910400671000_2022.11.17.516888v1.15"
},
{
"DOI": "10.1016/j.medj.2022.09.010",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.16",
"unstructured": "Planas, D. , et al. Duration of BA.5 neutralization in sera and nasal swabs from SARS-CoV-2 vaccinated individuals, with or without omicron breakthrough infection. Med (2022)."
},
{
"DOI": "10.1101/2022.10.31.514580",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.17",
"unstructured": "Kurhade, C. , et al. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1, and XBB.1 by 4 doses of parental mRNA vaccine or a BA.5-bivalent booster. Biorxiv, 2022.2010.2031.514580 (2022)."
},
{
"DOI": "10.1101/2022.10.19.512891",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.18",
"unstructured": "Qu, P. , et al. Distinct Neutralizing Antibody Escape of SARS-CoV-2 Omicron Subvariants BQ.1, BQ.1.1, BA.4.6, BF.7 and BA.2.75.2. Biorxiv, 2022.2010.2019.512891 (2022)."
},
{
"DOI": "10.1101/2022.09.16.508299",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.19",
"unstructured": "Sheward, D.J. , et al. Omicron sublineage BA.2.75.2 exhibits extensive escape from neutralising antibodies. Biorxiv, 2022.2009.2016.508299 (2022)."
},
{
"DOI": "10.1016/S1473-3099(22)00694-6",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.20",
"unstructured": "Wang, Q. , et al. Resistance of SARS-CoV-2 omicron subvariant BA.4.6 to antibody neutralisation. The Lancet Infectious Diseases (2022)."
},
{
"DOI": "10.1056/NEJMc2212117",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.21",
"unstructured": "Hachmann, N.P. , Miller, J. , Collier, A.-r.Y. & Barouch, D.H. Neutralization Escape by SARS-CoV-2 Omicron Subvariant BA.4.6. New England Journal of Medicine (2022)."
},
{
"DOI": "10.1101/2022.11.01.514722",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.22",
"unstructured": "Miller, J. , et al. Substantial Neutralization Escape by the SARS-CoV-2 Omicron Variant BQ.1.1. Biorxiv, 2022.2011.2001.514722 (2022)."
},
{
"DOI": "10.1101/2022.09.15.507787",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.23",
"unstructured": "Cao, Y. , et al. Imprinted SARS-CoV-2 humoral immunity induces converging Omicron RBD evolution. Biorxiv, 2022.2009.2015.507787 (2022)."
},
{
"article-title": "Imprinted antibody responses against SARS-CoV-2 Omicron sublineages",
"first-page": "eadc9127",
"journal-title": "Science",
"key": "2022111910400671000_2022.11.17.516888v1.24",
"volume": "0",
"year": "2022"
},
{
"DOI": "10.1101/2022.10.24.513619",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.25",
"unstructured": "Collier, A.-r.Y. , et al. Immunogenicity of the BA.5 Bivalent mRNA Vaccine Boosters. Biorxiv, 2022.2010.2024.513619 (2022)."
},
{
"DOI": "10.1101/2022.10.31.514636",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.26",
"unstructured": "Davis-Gardner, M.E. , et al. mRNA bivalent booster enhances neutralization against BA.2.75.2 and BQ.1.1. Biorxiv, 2022.2010.2031.514636 (2022)."
},
{
"DOI": "10.1101/2022.10.22.513349",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.27",
"unstructured": "Wang, Q. , et al. Antibody responses to Omicron BA.4/BA.5 bivalent mRNA vaccine booster shot. Biorxiv, 2022.2010.2022.513349 (2022)."
},
{
"DOI": "10.1093/dnares/dsu029",
"doi-asserted-by": "publisher",
"key": "2022111910400671000_2022.11.17.516888v1.28"
},
{
"DOI": "10.1099/jgv.0.001453",
"article-title": "SARS-coronavirus-2 replication in Vero E6 cells: replication kinetics, rapid adaptation and cytopathology",
"doi-asserted-by": "crossref",
"first-page": "925",
"journal-title": "Journal of General Virology",
"key": "2022111910400671000_2022.11.17.516888v1.29",
"volume": "101",
"year": "2020"
},
{
"DOI": "10.1038/s41586-022-04474-x",
"article-title": "Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity",
"doi-asserted-by": "crossref",
"first-page": "706",
"journal-title": "Nature",
"key": "2022111910400671000_2022.11.17.516888v1.30",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1016/j.chom.2022.09.018",
"article-title": "Characterization of the enhanced infectivity and antibody evasion of Omicron BA.2.75",
"doi-asserted-by": "crossref",
"first-page": "1527",
"journal-title": "Cell Host & Microbe",
"key": "2022111910400671000_2022.11.17.516888v1.31",
"volume": "30",
"year": "2022"
},
{
"DOI": "10.1038/s41591-022-01816-0",
"article-title": "Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2",
"doi-asserted-by": "crossref",
"first-page": "1491",
"journal-title": "Nature Medicine",
"key": "2022111910400671000_2022.11.17.516888v1.32",
"volume": "28",
"year": "2022"
},
{
"article-title": "Characterization of a human ovarian adenocarcinoma line, IGROV1, in tissue culture and in nude mice",
"first-page": "4970",
"journal-title": "Cancer Res",
"key": "2022111910400671000_2022.11.17.516888v1.33",
"volume": "45",
"year": "1985"
},
{
"DOI": "10.15252/embj.2020106267",
"doi-asserted-by": "publisher",
"key": "2022111910400671000_2022.11.17.516888v1.34"
},
{
"key": "2022111910400671000_2022.11.17.516888v1.35",
"unstructured": "Planas, D. , et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nature Medicine (2021)."
},
{
"DOI": "10.1016/j.immuni.2022.05.005",
"article-title": "Antibody-mediated neutralization of SARS-CoV-2",
"doi-asserted-by": "crossref",
"first-page": "925",
"journal-title": "Immunity",
"key": "2022111910400671000_2022.11.17.516888v1.36",
"volume": "55",
"year": "2022"
},
{
"DOI": "10.1038/s41577-021-00542-x",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.37",
"unstructured": "Taylor, P.C. , et al. Neutralizing monoclonal antibodies for treatment of COVID-19. Nature Reviews Immunology (2021)."
},
{
"DOI": "10.1056/nejmoa2109682",
"doi-asserted-by": "publisher",
"key": "2022111910400671000_2022.11.17.516888v1.38"
},
{
"DOI": "10.1056/NEJMoa2116620",
"article-title": "Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for Prevention of Covid-19",
"doi-asserted-by": "crossref",
"first-page": "2188",
"journal-title": "New England Journal of Medicine",
"key": "2022111910400671000_2022.11.17.516888v1.39",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "New England Journal of Medicine",
"key": "2022111910400671000_2022.11.17.516888v1.40",
"volume": "385",
"year": "2021"
},
{
"key": "2022111910400671000_2022.11.17.516888v1.41",
"unstructured": "WHO. Therapeutics and COVID-19: living guideline. Sept 16, 2022 Update https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.5 (2022)."
},
{
"key": "2022111910400671000_2022.11.17.516888v1.42",
"unstructured": "NIH. COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/ (2022)."
},
{
"DOI": "10.1016/j.celrep.2022.110812",
"article-title": "LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants",
"doi-asserted-by": "crossref",
"first-page": "110812",
"journal-title": "Cell Reports",
"key": "2022111910400671000_2022.11.17.516888v1.43",
"volume": "39",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(22)00495-9",
"article-title": "A monoclonal antibody stands out against omicron subvariants: a call to action for a wider access to bebtelovimab",
"doi-asserted-by": "crossref",
"first-page": "1278",
"journal-title": "The Lancet Infectious Diseases",
"key": "2022111910400671000_2022.11.17.516888v1.44",
"volume": "22",
"year": "2022"
},
{
"key": "2022111910400671000_2022.11.17.516888v1.45",
"unstructured": "France, S.p. Point épidémiologique COVID-19 du 10 février 2022. https://www.santepubliquefrance.fr/presse/2022/point-epidemiologique-covid-19-du-10-fevrier-2022-le-ralentissement-de-la-circulation-du-sars-cov-2-se-confirme-et-s-accompagne-d-une-baisse-des (2022)."
},
{
"DOI": "10.1038/s41586-022-04462-1",
"article-title": "Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant",
"doi-asserted-by": "crossref",
"first-page": "700",
"journal-title": "Nature",
"key": "2022111910400671000_2022.11.17.516888v1.46",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1038/s41591-022-01792-5",
"article-title": "Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies",
"doi-asserted-by": "crossref",
"first-page": "1297",
"journal-title": "Nature Medicine",
"key": "2022111910400671000_2022.11.17.516888v1.47",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2022.09.018",
"article-title": "Virological characteristics of the SARS-CoV-2 Omicron BA.2 subvariants, including BA.4 and BA.5",
"doi-asserted-by": "crossref",
"first-page": "3992",
"journal-title": "Cell",
"key": "2022111910400671000_2022.11.17.516888v1.48",
"volume": "185",
"year": "2022"
},
{
"DOI": "10.1016/j.chom.2022.10.003",
"article-title": "Virological characteristics of the SARS-CoV-2 Omicron BA.2.75 variant",
"doi-asserted-by": "crossref",
"first-page": "1540",
"journal-title": "Cell Host & Microbe",
"key": "2022111910400671000_2022.11.17.516888v1.49",
"volume": "30",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(22)00591-6",
"article-title": "Lung cell entry, cell-cell fusion capacity, and neutralisation sensitivity of omicron sublineage BA.2.75",
"doi-asserted-by": "crossref",
"first-page": "1537",
"journal-title": "The Lancet Infectious Diseases",
"key": "2022111910400671000_2022.11.17.516888v1.50",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1038/s41564-020-00789-5",
"article-title": "Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies",
"doi-asserted-by": "crossref",
"first-page": "1185",
"journal-title": "Nature Microbiology",
"key": "2022111910400671000_2022.11.17.516888v1.51",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1101/2022.08.12.22278699",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.52",
"unstructured": "Bruel, T. , et al. Longitudinal analysis of serum neutralization of SARS-CoV-2 Omicron BA.2, BA.4 and BA.5 in patients receiving monoclonal antibodies. medRxiv, 2022.2008.2012.22278699 (2022)."
},
{
"DOI": "10.1016/j.jinf.2022.06.033",
"article-title": "Sotrovimab to prevent severe COVID-19 in high-risk patients infected with Omicron BA.2",
"doi-asserted-by": "crossref",
"first-page": "e104",
"journal-title": "J Infection",
"key": "2022111910400671000_2022.11.17.516888v1.53",
"volume": "85",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)01938-9",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.54",
"unstructured": "Wu, M.Y. , et al. WHO’s Therapeutics and COVID-19 Living Guideline on mAbs needs to be reassessed. The Lancet (2022)."
},
{
"DOI": "10.1126/science.adc9127",
"article-title": "Imprinted antibody responses against SARS-CoV-2 Omicron sublineages",
"doi-asserted-by": "crossref",
"first-page": "619",
"journal-title": "Science",
"key": "2022111910400671000_2022.11.17.516888v1.55",
"volume": "378",
"year": "2022"
},
{
"DOI": "10.1038/s41591-022-02092-8",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.56",
"unstructured": "Scheaffer, S.M. , et al. Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant in mice. Nature Medicine (2022)."
},
{
"DOI": "10.1038/s41591-022-02031-7",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.57",
"unstructured": "Chalkias, S. , et al. Safety, immunogenicity and antibody persistence of a bivalent Beta-containing booster vaccine against COVID-19: a phase 2/3 trial. Nature Medicine (2022)."
},
{
"DOI": "10.1038/s41591-022-01877-1",
"article-title": "Rapid evaluation of COVID-19 vaccine effectiveness against symptomatic infection with SARS-CoV-2 variants by analysis of genetic distance",
"doi-asserted-by": "crossref",
"first-page": "1715",
"journal-title": "Nature Medicine",
"key": "2022111910400671000_2022.11.17.516888v1.58",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1126/science.abq1841",
"doi-asserted-by": "publisher",
"key": "2022111910400671000_2022.11.17.516888v1.59"
},
{
"DOI": "10.1016/j.cell.2021.03.028",
"article-title": "N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2",
"doi-asserted-by": "crossref",
"first-page": "2332",
"journal-title": "Cell",
"key": "2022111910400671000_2022.11.17.516888v1.60",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1126/scitranslmed.abn3715",
"doi-asserted-by": "publisher",
"key": "2022111910400671000_2022.11.17.516888v1.61"
},
{
"DOI": "10.1016/j.cell.2022.09.032",
"article-title": "Interval between prior SARS-CoV-2 infection and booster vaccination impacts magnitude and quality of antibody and B cell responses",
"doi-asserted-by": "crossref",
"first-page": "4333",
"journal-title": "Cell",
"key": "2022111910400671000_2022.11.17.516888v1.62",
"volume": "185",
"year": "2022"
},
{
"DOI": "10.1016/j.ebiom.2021.103637",
"doi-asserted-by": "crossref",
"key": "2022111910400671000_2022.11.17.516888v1.63",
"unstructured": "Monel, B. , et al. Release of infectious virus and cytokines in nasopharyngeal swabs from individuals infected with non-alpha or alpha SARS-CoV-2 variants: an observational retrospective study. eBioMedicine 73(2021)."
}
],
"reference-count": 63,
"references-count": 63,
"relation": {},
"resource": {
"primary": {
"URL": "http://biorxiv.org/lookup/doi/10.1101/2022.11.17.516888"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies",
"type": "posted-content"
}
